Chlorobium FMO antenna complex characterisation

Characteristics of Green Sulfur Bacteria
In the absence of light, life on earth would not exist. Biological photosynthesis is among the most important reactions on planet earth that allows life to thrive. It was long thought that life was restricted primarily to photic zones where photosynthetic primary producers could access the light needed to drive their metabolism. This view was challenged with the discovery of green sulfur bacteria that grow deep in the ocean floor. Green sulfur bacteria are photosynthetic microbes that are capable of thriving in ecological regions that have extremely low levels of solar radiation. It has been proposed that certain types of green sulfur bacteria use what is known as Geothermal Radiation to supplement their chemotrophic metabolism. The emission of photons from geothermal vents could have provided a selective advantage to chemotrophic ancestors of modern day green sulfur bacteria that utilized light sensing systems to perform phototaxis towards sulfur-rich geothermal vents (3). The discovery of a new microbial world at the bottom of the ocean contributes to our knowledge of the incredible biological diversity that our planet planet nurtures.
On Earth, the discovery of a photosynthetic species capable of using geothermal radiation, rather than solar sources, helps support the hypothesis that life may exist on other planets devoid of star-light (10). In addition to providing supporting evidence for extraterrestrial life, it is speculated by some that life on Earth originated in conditions similar to those displayed in the vicinity surrounding deep sea hydrothermal vents (11).
In green sulfur bacteria, the harvesting of light is in part carried out by the Fenna-Matthews-Olson protein (FMO). Based on the current literature, the FMO protein is thought to belong uniquely to the green sulfur bacteria (4). The chlorobiaceae contain Fe-S type reaction centers that share an ancestor with oxygenic photosynthetic organisms that utilize photosystem I (4).
Discovery of the Fenna-Matthews-Olson Protein Membrane Orientation in Chlorobaculum tepidum
A team of researchers at Washington University in St. Louis, lead by Robert Blankenship, has pioneered a new method of discovering protein orientation in living systems (1). By combining chemical labeling with mass spectroscopy, these scientists brought forth knowledge of the structure/function relationship of the Fenna-Matthews-Olson (FMO) protein in Chlorobaculum tepidum. C. tepidum is a member of the Chlorobium genera and are closely related, yet distinct from, the Bacteroides genera (2). As a result of the research lead by R. Blankenship, and other equally important contributions in the literature, there is a general consensus that the FMO complex consists of seven BChl a pigment molecules, as well as an eighth pigment, that are arranged within the FMO protein complex in a such a manner to form a hetero-trimeric structure that exhibits C-3 symmetry. The axial of symmetry is believed to be perpendicular to the cytoplasmic membrane and this general orientation has not yet been disputed. Unfortunately, the horizontal orientation of the C-3 FMO disc is not yet agreed upon.
Introduction to the FMO protein
In C. tepidum, the Fenna-Matthews-Olson protein is part of a light-harvesting complex that consists of three major components: 1. Chlorosome 2. Aforementioned FMO protein, and 3. Reaction center. This system captures electromagnetic radiation from the sun and forms a funnel-like system that directs energy transfer (3). The role of the FMO protein is analogous to that of a wire connecting two electrodes. That is to say that the FMO protein provides the transfer of energy from the chlorosome to the reaction center.
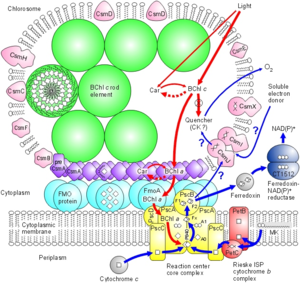
The Fenna-Matthews-Olson protein complex has been identified in all surveyed green sulfur bacteria to date (4). During the late 1970’s, the FMO complex was first described in the organism Prosthecochloris aestuarii (5, 6). The FMO protein complex contains pigments that molecular biologists refer to as bacteriochlorophyll proteins. In the FMO complex, seven bacteriochlorophyll proteins are present (BChl a). The FMO complex has a hetero-trimeric structure that has been resolved at a maximum resolution of 1.9 angstroms (7). With this high resolution, knowledge of the structure has provided a background for the optical analysis of the protein. However, as is the case with many advancing work in science, the theoretical framework for this optical analysis has been disputed by other researchers (8). More recent work has been done to elucidate both the structure of the FMO complex and its orientation in relation to the cell membrane, the cytoplasmic membrane, and the reaction center (4). Each of the three monomers in the FMO complex has a mass of 40 kDa and form 3-fold symmetry (4). Together, these three monomers form C-3 symmetry and have an axis that is thought to be perpendicular to the cellular membrane. Each of the three monomers contains seven of the BChl a molecules in addition to another less characterized eighth pigment (12, 13). The BChl a protein plays a critical role in transferring light energy to the reaction complex. It has been proposed that the BChl a molecules function through excitons of individually excited molecules (4, 6). The strength of the interactions of these excitons depends on the orientation of the BChl a proteins, as well as the distance between them (4, 6). Recently discovered quantum effects of within the FMO complex are thought to provide more efficient energy transfer as the light energy travels from the cytoplasmic membrane to the reaction center (14, 15, 16). Researchers have assigned numbers to the individuals BChl a monomers to help with communication aimed towards verifying the orientation of the FMO complex. For example, BChl a #3 (named so for historical reasons) is thought to have the lowest site energy. This suggests that the FMO complex is oriented such that BChl a#3 is opposite of the reaction center and facing towards the cytoplasmic membrane. Converse to the evidence in support of this theory are the results of research that examined the structure and properties of the isolated protein. Investigations of the hydrophobicity of the protein suggest that BChl a# 1 is facing the the cytoplasmic membrane (4). This orientation model is the exact opposite of that suggested by Blankenship et al. (9). Methods of studying the orientation of the FMO complex have included linear dichroism (17) and 3D reconstructions made possible by STEM imaging (18). These two methods have been useful in suggesting that the FMO disc flat with its C3 axis of symmetry perpendicular to the cytoplasmic membrane, but they are not insightful with regards to which side of the FMO disc actually contacts or faces the cytoplasmic membrane. Other details of the overall antenna complex remain unclear, as well (4). Within the chlorosome, the interaction of the Csma protein with the FMO disc is not clear but there is evidence to suggest that Csma and the FMO trimer are in direct contact and that, perhaps, the FMO complex is partially submersed within a layer of Csma proteins (19). Zhou, Blankenship et al. (1997) asked whether or not the complex is embedded in the cellular membrane by examining the assemblage of amino acids in the trimer (4). They found that the top surface of the BChl a trimer has mostly hydrophilic amino acid residues and that the bottom surface has relatively more hydrophobic residues. According to their analysis, up to forty-percent of the BChl a trimer may be embedded in the membrane. Furthermore, this evidence is in agreement with the linear dichroism studies conducted on the BChl a protein isolated from P. aestuarii and C. tepidum (20).
Optical Properties of the Fenna-Matthews-Olson Complex
Because the FMO complex serves to transfer optical energy to the reaction center, the optical properties of the complex are important for adequate function of the photosynthetic system. Blankenship et al. have summarized the results of spectroscopic investigations that have been conducted on the BChl a protein isolated from P. aestuarii and C. tepidum (21). The BChl a protein is homologous between the two species. However, the results of circular dichroism spectra and high-pressure hole-burning studies of the protein revealed that excitonic interactions between BChl a molecules in the two homologs are not identical (21, 22). In C. tepidum, the range of low-temperature Qy absorption bands of BChl a is approximately 240 cm-1 more broad than that of the P. aestuarii homolog. It is speculated that the discrepancy in this interaction is due to different arrangements of the tertapyrroles, or differences in the BChl-protein interactions (4). Otherwise, the optical properties of the two proteins are generally similar.
The light absorption profile of a recently discovered green sulfur bacterium that lives around a deep-sea hydrothermal vent reveals an in vivo fluorescence emission peak at ≈775 nm (3, Figure 3). This peak indicates the presence of the BChl c protein monomer. Quantitatively, this was the major pigment in this previously unknown bacterium. This major peak at ≈775 nm is not far from other reports of the BChl a absorption peak that occurs ≈800 nm (16).
Relating the Light Harvesting Systems of Other Species to the BChl a Protein Structure in Green Sulfur Bacteria
The structures of light-harvesting complexes from several species have been reported in the literature during the past fifteen years. In particular, chlorophyll a/b-protein complexes from higher plants (21), light-harvesting complex II from Rhodopseudomonas acidophila (22) and Rhodospirillum molishianum (23), and the Peridinin-chlorophyll-protein utilized by Amphidinium cartera (24) have been studied. Interestingly, the bacteriochlorophyll a proteins examined have been shown to be peripheral membrane proteins while the light-harvesting chlorophyll a/b (LHC) and light-harvesting complex II (LHC2) systems are integral membrane proteins. A unifying characteristic of all studied light-harvesting complexes is the symmetrical assembly of subunits and all complexes examined so far have either trimeric or ring structures (4). It is thought that by having symmetrical and repeating subunits, the complexes can have more efficient energy transfer and benefit from the higher structural integrity associated with identical repeating subunits (4). The repetition of identical subunits also helps keep the complex properly oriented with the membrane without requiring long poly-peptides. All light-harvesting complexes require cofactors that act to localize energy. Tetrapyrroles present in the harvesting complexes vary in number between six and 36 (4). Some complexes (LHC, LH2, PCP) have, in addition to tetrapyrroles, carotenoids such as lutein (LHC), rhodopsin-glucoside (LH2 of R. acidophila) and lycopene (LH2 of R. molischianum). Carotenoids are useful not only in providing a greater range of absorption wavelengths, but also in quenching triplet-states of chlorophyll and bacteriochlorophylls. The latter of these two functions provide photoprotection and structural maintenance of the complex (4).
A significant variation in the secondary structure of light-harvesting complexes exists. This variation can be summarized by breaking the harvesting complexes down into two groups: ones that consist of alpha-helical secondary structures, and ones that consist of beta-sheet secondary structures (4). As a reference, the BChl a protein is comprised of a beta-sheet secondary structure. It is possible that the beta-sheet secondary structure of BChl a allows for a more compact arrangement of several pigment molecules. For integral proteins, the alpha-helical secondary structure provides a short and structurally robust form for insertion into the membrane.
Relationships of the FMO Complexes in C. tepidum and P. aestuarii
Comparison of the amino acid residue sequences of the BChl a protein in C. tepidum and P. aestuarii show seventy-eight percent homology (4). In P. aestuarii, there is an extra Asn amino acid between Ala174 and Phe175 of C. tepidum. Although there is only a single extra residue, helix 2 of P. aestuarii is elongated in comparison to helix 2 of C. tepidum. The secondary structure of the two homologs are very similar. Only eighty residues are different between the two and the locations of these mismatches appear to be either on the surface or in the core of the protein. These eighty differential residues do not appear to affect the interactions of the BChl a proteins (4). Superimposing the tetrapyrroles onto one another show that the shape of the BChl proteins are largely unchanged.
One of the most important parameters affecting energy transfer within the FMO complex is the distance between BChl monomers (4). Between P. aestuarii and C. tepidum, this distance is highly conserved. Each comparative pairwise analysis of the distance between BChl monomers in P. aestuarii and C. tepidum shows that the largest difference between the species is no greater than 0.3 angstroms (4). The position of the Mg atom in relation to the position of the bacteriochlorin ring is a useful measure planarity. The average Mg atom displacement between P. aestuarii and C. tepidum is only 0.09 angstroms, further proving the high homology of the two BChls.
Recent Discovery of Green Sulfur Bacteria on From a Deep Sea Hydrothermal Vent
The work of Beatty, Blankenship, et al. (2005) lead to the discovery of a previously undiscovered species that lives in the near vicinity of a deep-sea hydrothermal vent in the East Pacific Rise (Figure 5). The region from which the microbe was extracted is spotted with volcanic sites and many different types of underwater vents, making the area ideal for discovering new photosynthetic anaerobes. Although it is possible to verify the presence of organisms by analyzing samples of bulk DNA, Beatty et al. took a different approach to discern the presence of intact cells. They took samples of ocean water for cultivation rather than direct PCR amplification of bulk DNA. Samples were grown in medium prepared specifically for the cultivation of green and purple photosynthetic bacteria. Incubation occurred at 25 degrees C and a weak fluorescent light, as well as a 60-watt incandescent bulb, was turned on to provide a source of photons. Samples were also grown in tubes containing salineSL10 solution. In these salineSL10 tubes, an increase in microbe growth was observed when acetate, elemental sulfur, propionate, or 0.05% peptone were added. One-hundred other substances were added to samples, but no such increase in growth was observed. Degenerate PCR was performed on DNA extracted by standard methods and 16S rRNA primers were used to conduct sequence analysis with previously identified and characterized organisms. This comparison was done using the NCBI database and ClustalX software. For further comparison, oligonucleotide primers GSB1 were used to amplify a 972 bp segment of DNA that encodes a 323 amino acid sequence. This sequence was found to be between seventy-one and ninety-one percent homologous with fourteen species of green sulfur bacteria. By contructing a phylogenetic tree, it was determined that the isolated colony is most closely related to Chlorobium and Prosthecochloris aquatic species(3, Figure 4).

Conclusion
The FMO protein complex plays a vital role in a photosynthetic organism, making it one of the most important protein complexes involved in primary production in deep-sea thermal ecosystems as well as other communities inhabited by green sulfur bacteria. While the complex was first studied more than thirty years ago, recent work has extended our knowledge of the complex molecular and atomic structure of the complex and its substituent molecules. Discoveries of new microbes are occurring that challenge previous conceptions of suitable habitats for bacteria. Some research has indicated that green sulfur bacteria survival has been enhanced through the utilization of geothermal radiation by the FMO complex. The critical role of the FMO complex is apparent from the high conservation of the complex across green sulfur bacteria. Fortunately, novel methods of investigation have been applied to the FMO complex and these new approaches are bringing scientists closer and closer to understanding the structure/function relationship that exists in this protein that is unique to the green sulfur bacteria.
References
(1)Washington University in St. Louis. "'Taco Shell' Protein: Orientation Of Antenna Protein In Photosynthetic Bacteria Described." ScienceDaily 9 April 2009. 12 April 2009 <http://www.sciencedaily.com¬ /releases/2009/04/090402171438.htm>.
( 2)D.A. Bryant & N.-U. Frigaard (November 2006). "Prokaryotic photosynthesis and phototrophy illuminated". Trends Microbiol. 14 (11): 488. doi:10.1016/j.tim.2006.09.001
(3)Beatty, J.T.; Overmann, J.; Lince, M.T.; Mansket, A.K.; Lang, A.S.; Blankenship, R.E.; Van Dover, C.L.; Martinson, T.A.; Plumley, F.G. “ An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent”. PNAS June 28, 2005 vol. 102 no. 26 9306-9310
(4)Li YF, Zhou W, Blankenship RE, Allen JP (1997) Crystal structure of the bacteriochlorophyll a protein from Chlorobium tepidum. J Mol Biol 271:456–471.
(5)Olson, J. M. (1978). Bacteriochlorophyll a-proteins from green bacteria. In The Photosynthetic Bacteria (Clayton, R. K. & Sistrom, W. R., eds), pp. 161± 178, Plenum Press, New York
(6)Olson, J. M., Ke, B. & Thompson, K. H. (1976). Exciton interaction among chlorophyll molecules in bacteriochlorophyll a proteins and bacteriochlorophyll a reaction center complexes from green bacteria. Biochem. Biophys. Acta, 430, 524±537.
(7)Tronrud, D. E. & Matthews, B. W. (1993). Refinement of the structure of a water-soluble antenna complex from green photosynthetic bacteria by incorporation of the chemically determined amino acid sequence. In The Photosynthetic Reaction Center (Norris, J. & Deisenhofer, J., eds), vol. 1, pp. 13±21, Academic Press, New York.
(8)Gülen, D. (1996). Interpretation of the excited-state structure of the Fenna-Matthews-Olson pigment protein complex of Prosthecochloris aestuarii based on the simultaneous simulation of the 4K absorption, linear dichroism, and singlet-triplet absorption difference spectra: a possible excitonic explanation?. J. Phys. Chem. 100, 17683±17689.
(9)Wen, J.; Zhang, H.; Gross, M.L.; Blankenship,, R.E. (2008) Membrane orientation of the FMO antenna protein from Chlorobaculum tepidum as determined by mass spectrometry-based footprinting. PNAS. (www.pnas.org_cgi_doi_10.1073_pnas.0901691106)
10)Chyba, C. F. & Hand, K. P. (2001) Life Without Photosynthesis. Science 292, 2026–2027
11)Martin, W. & Russell, M. J. (2003). On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. London B 358, 59–85.
12)Ben-Shem A, Frolow F, Nelson N (2004) Evolution of Photosystem 1—from symmetry through pseudosymmetry to asymmetry. FEBS Lett 564:274–280.
13)Tronrud DE, Wen JZ, Gay L, Blankenship RE (2009) The structural difference in absorbance spectra for the FMO protein from green sulfur bacteria (submitted).
14)Read EL, et al. (2008) Visualization of excitonic structure in the Fenna-Matthews-Olson photosynthetic complex by polarization-dependent two-dimensional electronic spectroscopy. Biophys J 95:847–856.
15)Mohseni M, Rebentrost P, Lloyd S, Aspuru-Guzik A (2008) Environment-assisted quantum walks in energy transfer of photosynthetic complexes. Quantum Physics arXiv:0805.2741v1.
16)Müh F, et al. (2007). α-Helices direct excitation energy flow in the Fenna-Matthews-Olson protein. Proc Natl Acad Sci USA 104:16862–16867.
17)Melkozernov AN, Olson JM, Li YF, Allen JP, Blankenship RE (1998) Orientation and excitonic interactions of the Fenna-Matthews-Olson Protein in membranes of the green sulfur bacterium Chlorobium tepidum. Photosynth Res 56:315–328.
18)Re´migy HW, et al. (1999) The reaction center complex from the green sulfur bacterium Chlorobium tepidum: A structural analysis by scanning transmission electron microscopy. J Mol Biol 290:851–858.
19) Li H, Frigaard NU, Bryant DA (2006) Molecular contacts for chlorosome envelope proteins revealed by cross-linking studies with chlorosomes from Chlorobium tepidum. Biochemistry 45:9095–9103.
20) Swarthoff, T., de Grooth, B. G., Meiburg, R. F., Rijgersberg, C. P. & Amesz, J. (1980). Orientation of pigments and pigment-protein complexes in the green photosynthetic bacterium Prosthecochloris aestuarii. Biochim. Biophys. Acta, 593, 51±59.
21) Blankenship, R. E., Olson, J. M. & Miller, M. (1995). Antenna complexes from green photosynthetic bacteria. In Anoxygenic Photosynthetic Bacteria (Blankenship, R. E., Madigan, M. T. & Bauer, C. E., eds), pp. 399±435, Kluwer Acad. Pub., Dordrecht.
22) Reddy, N. R. S., Jankowiak, R. & Small, G. J. (1995). High-pressure hole-burning studies of the bacteriochlorophyl a antenna complex from Chlorobium tepidum. J. Phys. Chem. 99, 16168±16178.
Edited by Khalid Eldahan , student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.



