Diagnosis and Prevention of Neisseria meningitides Induced Meningitis
By Emily Staudenmaier
Introduction
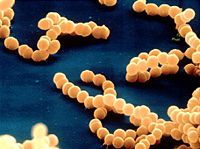
Meningitis is an inflammation of the membrane surrounding the human brain and spinal cord also known as the meninges (Figure 2). Meningitis can be caused by a bacterial or viral infection. Of the two types of infections, bacterial causation generally results in a more severe infection. The inflammation of the meninges results in headache, neck stiffness, fever, sensitivity to light and sometimes vomiting 5. These symptoms can progress quickly when bacteria are behind the infection, resulting in death 10-20% of the time 7. Three species of bacteria, Streptococcus pneumonia, Haemophilus influenzae and Neisseria meningitidis (Figure 1), are known to cause this infection. Certain characteristics of the latter species, N. meningitidis, result in difficult obstacles when it comes to the development of measures to diagnose, treat and prevent meningitis. Those obstacles and promising solutions are discussed in detail below.
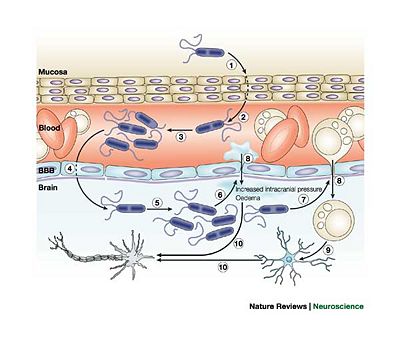
Neisseria meningitidis is Gram-negative, aerobic, nonmotile, coccal bacteria which is pathogenic only to Homo sapiens. Initial infection occurs in the nasopharynx where the bacteria can bind easily to the mucus membrane using their type IV pilli. These bacteria also have a capsid which is minimized during attachment to epithelial cells and then maximized to protect the organism agains the host's immune response. When present in the nasopharynx, the bacteria typically do not cause serious health problems to the host. In fact, 5-10% of adults have N. meningitidis present in their nasopharynx and do not develop meningitis 7. The asymptomatic presence of the bacteria can be beneficial to the human host since it allows antibodies to develop that can be useful in fighting a more serious subsequent infection. The entrance of the bacteria into the bloodstream results in serious consequences for the host. N. meningitidis has the unusual ability to cross the blood-brain barrier and enter the cerebral spinal fluid 7. The mechanism of this infiltration is unknown. Once the crossing has occurred, the bacteria release inflammatory cytokines which leads to the inflammation of the meninges (Figure 3).
Infection is spread through direct contact with nasal or oral fluids contaminated with the bacteria. Inhalation of large droplet nuclei can also result in the spread of N. meningitidis. N. meningitidis induced infections are most common and most serious in children, young adults and the elderly. In newborns and very young children, antibodies are transferred from the mother to the child via gestation and lactation. Soon after this contact between mother and child is severed, bactericidal activity decreases since the passive transfer of maternal antibodies no long occurs. Through exposure to asymptomatic colonization by bacteria, children are able to develop their own antibodies specific to these bacteria. Children who are no longer receiving antibodies from their mother and have yet to gain exposure to asymptomatic infections of the bacteria in their nasopharynx, lack any bactericidal immune response to the presence of N. meningitidis 4. Young children are therefore the most susceptible age group to contracting meningitis. Many vaccines and medications normally prescribed to fight an N. meningitidis infection are not recommended for children younger than two, which further complicates the issue of preventing and curing infection in this age group. Infection rate increases again for the young adult age group due simply to an increased level of exposure and transmission especially on college campuses where people live closely in relatively small spaces. The infection rate is also high in the elderly population. The elderly generally possess weakened immune systems which cause them to be more prone to infection.
N. meningitidis is also unique in that it can cause both epidemic and endemic cases of meningitis. Epidemic outbreaks of meningitis have become a serious issue in developing nations where the supply of vaccines and medication is limited and confined living situations result in increased transmission of virulent strains of the bacteria. One region of Africa has been termed the “Meningitis Belt”. The infection rate in this area can be as high as 1000 cases for every 100,000 people in the population 7.
Due to the highly infectious nature of virulent N. meningitidis and the quick escalation of the severity meningitis infections, timely diagnostic measures and effective vaccines are essential.
Important Features of N. meningitidis
Certain genetic and membranous features of N. meningitidis are vital to understanding and classifying strains of the bacteria. Outer membrane proteins, for example, are particularly important because they provide specific substrates for vaccines and medicines to target.
The main manner in which N. meningitidis is classified is by serogroup. A serogroup is defined by the presence of specific capsular polysaccharides (Figure 4). N. meningitidis strains have been classified into twelve different serogroups, however only five of the twelve are known to cause meningitis 6. The serogroups of the virulent strains are group A, B, C, Y and W-135. Vaccines exist against all of these serogroups except for serogroup B since its capsular polysaccharides are poorly immunogenic 2. Each serogroup causes a unique type of infection. For example, serogroup A tends to result in epidemic meningitis infections while serogroup B is typically behind endemic ones 6.
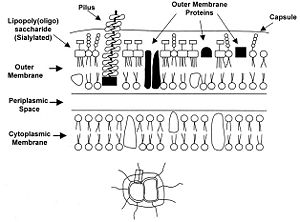
As far as genomes go, N. meningitidis has a fairly small one compared to other microorganisms such as E. coli. Their genome is approximately 2, 200 kb long and is particularly susceptible to mutation events due to multiple repetitive sequences and polymorphic sequences 7. During DNA replication, the replication machinery can slip on particularly long repetitive sequences which results in daughter DNA having either a larger or shorter number of repeats increasing variability in the next generation. Despite the fact that repetitive sequences are a prominent feature of N. meningitidis’ genome, the mutation rate is not particularly high (approximately 10-3 mutations per bacterial cell per generation), and no error-prone repair system exists which also decreases the occurrence and frequency of mutation 6. The majority of strain diversity is introduced by recombination.
N. meningitidis also has the interesting attribute of being naturally competent making it easy for the organism to acquire genetic information from other N. meningitidis cells. Competence has allowed for the rapid development of anti-biotic resistant strains which has created a serious barrier to treatment of meningitis. Strains resistant to penicillin, sulfa antibiotics and chloramphenicol have recently been discovered, which is particularly discouraging news since penicillin was a primary method of treatment, especially in epidemic outbreaks of meningitis 7. The competence of these organisms also allows them the frustrating ability to switch serogroups even during the course of an epidemic 6. Since vaccines are targeted to specific serogroups and certain treatments are more effective for some serogroups rather than others, the ability to switch which capsular polysaccharides are expressed makes treatment and prevention difficult. Therefore vaccines protecting against more than one serogroup have been developed but this still leaves the issue of antibiotic resistance.
Further proof of the variability of the genome can be seen by performing a phylogenetic analysis on various N. meningitidis sequences. After analyzing housekeeping genes which are fairly conserved among meningococci, a low level of congruence was observed which means that meningococcal genes are associated randomly 6. This randomness can be attributed to the natural competence of the bacteria which allows for frequent gene transfer.
Gene regulation in N. meningitidis can be used to optimize virulence. The capsule can shorten the amount that the pili extend from the cell. Since the pili are essential for binding the epithelial cells, a key step to colonization for both virulent and non-virulent strains, capsule proteins are down regulated during attachment 6. Attachment of the bacteria to the cell signals virulent strains to begin up-regulating expression of capsule proteins again since the presence of the capsule helps fend of bactericidal agents from the host’s immune system. Without the capsule, the bacteria are likely to die once it crosses into the blood stream 6. The careful coordination of gene expression levels of capsule and pili genes maximize virulence and transmission of N. meningitidis.
Detection and Diagnostic Techniques
Due to the quick rate of severity of meningitis infections, early detection of N. meningitidis and identification of which strain of the bacteria is behind the infection is important. When a patient presents with symptoms of meningitis, cultures of their cerebrospinal fluid are taken via a lumbar puncture and analyzed for indicators that bacteria may have infiltrated the blood-brain barrier. An increase in cerebrospinal fluid pressure, an increased protein content, decreased glucose content or the presence of white or red blood cells are all indicators that the patient has bacterial meningitis 1. In order to decide on the most effective course of treatment, as well as determine the susceptibility of the microbe to antibiotics, the specific species and serogroup of the bacteria must be identified which is achieved through culture-based biochemical tests. Although bacteria can sometimes be seen in cerebrospinal fluid under a microscope, species specificity is determined by growing cultures taken from the patient’s blood or cerebrospinal fluid 8. These culture-based tests can typically take 48-72 hours to complete which makes the tests are therefore dangerously long since time is of the essence when it comes to treating meningitis. 3.
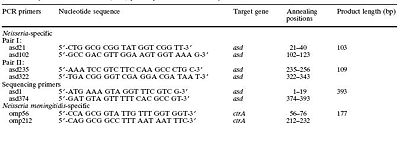
Luckily, researchers have developed much speedier methods to rapidly identify N. meningitidis. The method developed by Lansac et. al., is based on the use of polymerase chain reaction. The polymerase chain reaction is based on priming specific DNA sequences of interest and amplifying them. Therefore, primers for genes specific to N. meningitidis were designed which was a difficult task in and of itself. Due to the highly variable nature of the organism's genome, it is difficult to choose a gene which will always be present in any N. meningitidis culture but whose sequence is specific only to the species of interest and not other bacteria. The researchers chose ctr A gene which codes for a specific outer membrane protein that is conserved among N. meningitidis strains and specific to the species (Figure 5).
After the primers are designed, a reaction including primers and DNA isolated from the unknown species are prepared with other reactants in a mixture which after a series of heating and cooling cycles results in the amplification of a sequence. Since the primers used in Lansac’s method are specific to N. meningitidis, if the DNA is from any other species the reaction will not occur and no sequence will be amplified. Lansac reported that the method effectively identified 9 of the 12 known serogroups and successfully amplified 321 out of 322 strains tested which is a very high success rate. The best part of this new diagnostic method is that it only takes 90 minutes to complete.
Vaccination Strategies
Due to the variable nature of N. meningitidis, many unique approaches to vaccination have been explored. However, vaccination has also raised issues about damaging natural flora in the nasopharynx or increasing susceptibility of the host to strains which can not be vaccinated against.
One approach in vaccine design is to target outer membrane protein (OMP) complexes. Typically the proteins targeted are class proteins which include PorA, PorB, or Opa, transferin binding proteins, or ferric binding protein A 4. These proteins are specific to certain serogroups, not universal throughout the entirety of the N. meningitidis species. Therefore, each OMP vaccine is able to protect against a specific serogroup and only that serogroup. In the specificity of the vaccine lays a major flaw to the approach. Since the bacteria are able to switch between serogroups due to the regularity with which they undergo horizontal gene transfer, the effectiveness of OMP vaccines can easily be compromised.
When vaccines containing OMPs specific to N. meningitidis are administered they work by inducing the production of protective antibodies against the bacteria 4. Other vaccine designs operate by eliciting a bactericidal response against the serotype it targets. These vaccines are called anti-meningococcal polysaccharidic vaccines.
Anti-meningococcal polysaccharidic vaccines carry with them their own set of benefits and setbacks. One benefit is that they can target multiple serotypes by creating mixtures of serogroup specific polysaccharide targets 4. Currently there are vaccines to target group A, the combination of groups A and C or the combination of groups A, C, Y and W-135 7. The lack of a vaccine against serogroup B is notable. Although immunity against multiple serotypes is a great benefit to this method, the immunity is short-lived. Typically immunity from these vaccines lasts only 3 years 7. While this amount of time is not ideal for people in areas where meningitis epidemics are common, it is generally enough time for the recipient of the vaccine to grow out of age groups that are most likely to contract to infection. So if this vaccine is administered to a young child it allows them time to develop their own immunity through exposure to asymptomatic colonizations of N. meningitidis in their nasopharynx. Similarly if the vaccine is administered at the beginning of a young adult’s college years, they typically are immune for the duration of their college career during which they are most exposed to the pathogen. This line of thought does not extrapolate to the elderly populations. Anti-meningococcal polysaccharidic vaccines also have low effectiveness in young children, the primary age group which contracts meningitis and the least able to fight the infection 4.
The relatively short period of induced immunity and the ineffectiveness in young children causes anti-meningococcal polysaccharidic vaccines to be a less than ideal choice when it comes to prevention of epidemic outbreaks of meningitis. These shortcomings of the strategy make routine immunization of areas prone to epidemic outbreaks impossible 8. Instead, outbreaks must be quickly treated with oily chloramphenicol, a drug which combats a wide range of microbial infections. Oil chloramphenicol is a long-acting drug which makes it ideal for populations who rarely have the opportunity to have medicine administered to them. After the administration of this drug, epidemic districts are vaccinated with a more modern vaccination strategy discussed below, called protein-polysaccharide conjugate vaccines. It is then ideal to also treat nearby districts, called alert districts, with the vaccine. This strategy is predicted to prevent up to 70% of meningitis cases 8.
Figure 6 provides stark evidence for how much more susceptible young children are to meningitis infection. Therefore it is of the upmost importance to develop a vaccination strategy to aid this age group in combating the infection. Since anti-meningococcal polysaccharidic vaccines have proven to be ineffective in young children, a different vaccination strategy, protein-polysaccharide conjugate vaccines, is employed.
Protein-polysaccharide conjugate vaccines are similar to anti-meningococcal polysaccharidic vaccines in that they are effective against combinations of serogroups. They can effectively protect against serogroups C, the combination of A and C, and the combination of A, C, Y and W-135. These vaccines work by conjugating the polysaccharide to protein which converts the polysaccharide to a thymus-dependent antigen. This conversion results in a response similar to OMP complex vaccines in that it enhances IgG anticapsular antibodies and memory B cells in order to build up the host’s immune response to the serogroup 7.
While development of effective vaccines is clearly important in preventing deadly epidemic outbreaks of meningitis, there are some issues which have researchers and doctors questioning the use of vaccinations in general. One argument is that vaccines typically kill all Neisseria bacteria, not just Neisseria meningitidis. Neisseria lactamica, a harmless member of the natural flora of the nasopharynx, are one species that is lost to the host when a vaccine is administered. This may not seem like a large issue, except that research has shown N. lactamica to induce an immune response against N. meningitidis infections 4.
Sánchez et. al., have traced the occurrence of colonization of the nasopharynx by various bacteria over a human’s life cycle and drawn some interesting conclusions about the interaction of N. lactamica and N. meningitidis. N. lactamica colonization rates are typically very low during the first two years of a person’s life, which is the key time period at which young children are susceptible to meningitis infections caused by N. meningitidis. As the rate of N. lactamica colonization increases, the occurrence of N. meningitidis colonization decreases as do subsequent meningitis infections. This pattern caused the Sánchez group to question if N. lactamica provide immunity against N. meningitidis infections.
Upon further investigation, Sánchez et. al., found that N. lactamica share cross-reactive antigens with meningococci and therefore exposure of the host to N. lactamica prior to N. meningitidis results in the production of antibodies effective against different serotypes and serogroups of N. meningitidis. Since it is difficult to ethically justify immunity experiments in humans, infant mice were used to isolate immune sera instead since they show a response to the N. meningitidis pathogen that is relatively equivalent to what is observed in humans. Experimental treatments of the mouse immune sera consisted of a series of three immunizations. The immunization series consisted of various combinations of immunization using N. lactamica, N. meningitidis and a boost strain of N. meningitidis M982. Sánchez et. al., found that when treated with two doses of N. lactamica followed by a boost strain immunization, almost 1/3 of the treated sera resulted in significant killing (greater than 50%) and around 60% of the treated sera showed above a 40% killing. Therefore, prior exposure to colonization by N. lactamica can greatly reduce the rate of colonization by the potentially pathogenic bacteria, N. meningitidis. Since N. lactamica and N. meningitidis share cross-reactive antigens, vaccination against N. meningitidis infections also results in the death of N. lactamica colonies which, in turn, causes the loss of immunity due to the colonization of those bacteria. The researchers did comment, however, that colonization by N. lactamica alone will not provide complete immunity; colonization by non-virulent N. meningitidis strains is also required for immunity against meningococcal specific antigens such as PorA.
Sánchez et. al., supported the pursuit of vaccines against N. meningitidis which target outer membrane vesicles (OMVs). This vaccination approach would protect against one specific N. meningitidis strain for which the vaccine was designed but would have no effect on N. lactamica colonization in the host due to the lack of a polysaccharide capsule in N. lactamica. Vaccines which target OMVs are currently in the testing phase of development 4.
Conclusion
Meningitis, especially when caused by N. meningitidis, is a challenging infection to treat and prevent. The genome of the bacteria is constantly changing due to horizontal gene transfer, changing lengths of repetitive gene sequences and mutation events. Also, proteins in the capsule and outer membrane are easily regulated by the organism as are the extension of pili necessary for attachment so targeting these structures when designing vaccines and antibiotics is a bit risky. The most accurate methods of identification of N. meningitidis infections in patients involve identifying specific genes present in the culture so again the variability of the genome presents a problem. Despite the difficulties inherent in the design of treatment and prevention measures, researchers have developed new vaccines which effectively protect against meningitis for all age groups.
The development of these vaccines is important now more than ever due to the frequency of meningitis epidemics in developing nation paired with the increasing number of antibiotic resistant N. meningitidis strains. If the effectiveness of antibiotics traditionally used to treat the infection is declining, then preventative measures will be the main approach used to stop deadly epidemics. Also development of timely methods of diagnosis has allowed doctors to begin treatment of meningitis quickly. Early diagnosis and treatment also help to halt the spread of the infection during epidemics. Therefore, despite the evolution of antibiotic resistant strains, science and medicine have also evolved in a constant battle to fight meningitis by gathering knowledge about the bacteria that causes it.
References
1 Center for Disease Control. “Laboratory Methods for the Diagnosis of Meningitis Caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenza”. 1998. p. 20.
2 Cripps, A., Foxwell, R., and Kyd, J., “Challenges for the development of vaccines against Haemophilus influenzae and Neisseria meningitides”. Current Opinion in Immunology. 2002. Volume 14. p. 553-557
3 Lansac, N., Picard, F. J., Ménard, C., Boissinot, M., Ouellette, M., Roy, P. H., and Bergeron, M. G. “Novel Genus-Specific PCR-Based Assays for Rapid Identification of Neisseria Species and Neisseria meningitidis”. European Journal of Clinical Microbiology and Infectious Diseases. 2000. Volume 19. p. 443-451.
4 Sánchez, S., Troncoso, G., Criado, M. T., and Ferreirós, C., “In vitro induction of memory-driven responses against Neisseria meningitidis by priming with Neisseria lactamica”. Vaccine. 2002. Volume 20. p. 2957-2963.
5 Slonczewski, J., and Foster, J. Microbiology: An Evolving Science. New York: W. W. Norton, 2009.
6 Taha, M. K., Deghmane, A. E., Antignac, A., Leticia Zarantonelli, M., Larribe, M., and Alonso, J. M. “The duality of virulence and transmissibility in Neisseria meningitidis”. Trends in Microbiology. 2002. Volume 10. p. 376-382.
7 Tzeng, Y. L. and Stephens, D. “Epidemiology and pathogenesis of Neisseria meningitides”. Microbes and Infection. 2000. Volume 2. p. 687-700.
8 The World Health Organization. “Meningococcal Meningitis”. 2003. http://www.who.int/mediacentre/factsheets/2003/fs141/en/
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.