Foot-and-Mouth Disease

Baltimore Classification
Group IV. Positive single-stranded RNA (picornavirus)
Higher order categories
Order Picrnovirales
Family Picornaviridae
Genus Aphthovirus
Species Bovine rhinitis virus
Description and Significance
This aphthovirus can survive a range of conditions outside of a host cell. It can persist in below-freezing temperatures as well as through the high temperatures of pasteurization, making the eradication of this virus in a cattle population and throughout its byproducts very difficult. Any meat or dairy products produced by infected herds is thereby contaminated and economic impacts of FMD are severe.
Herds are quickly affected with FMD due to their close contact with each other and materials that carry the virus. Cloven-hoofed livestock such as cows, pigs, sheep, goats, and sometimes llama and aplaca herds are the most often affected animals. Outbreaks of FMD have not occurred in the United States since the late 1920's [3] but Asia, Africa, and regions in the Middle East still suffer. Animals can carry the virus for two or three days before they show symptoms, and afterwards for up to four years, in some cases [1].
It's highly contagious nature as well as it's relation to the human-affecting enterovirus that causes hand, foot, and mouth disease make it a model virus to study. If infected animals come into contact with wild herds, deer and buffalo will also become diseased and sources of food and ecological balance will be rendered dangerous. The fact that an infection can cause immunity to that particular serotype but not to any of the others is an alarming dilemma that vaccinators must try and quell; we can learn much about the possibility of a blanket vaccination that is effective for all strands of a virus.
Genome Structure
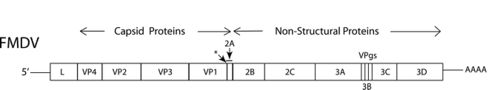
The FMDV genome consists of about 8,200 nucleotides, larger than many other picornavirus genuses [2]. There are seven different strains of the virus: A, O, C, Asia 1, SAT 1, SAT 2, and SAT 3. Immunity to one of these serotypes does not guarantee immunity to any of the others [2] [3].
Like most picornaviruses, it is a single strand of positive-sense RNA. P1 of the genome encodes for the capsid proteins VP1-4, while P2/3AB and 3CD are regulatory.
Virion Structure of FMD virus
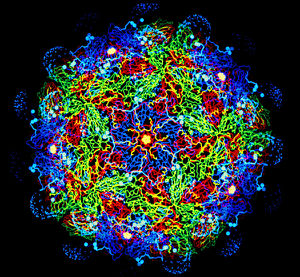
The FMD virus' capsid is non-enveloped and icosahedral in shape, made up of VP1, VP2, and VP3 on the outer side of the shell and coated on the inside with VP4. The VP1 protein forms a long loop that contains a peptide sequence of Arg-Gly-Asp that is thought to play a role in receptor binding [6].
A strand of positive-sense RNA inhabits the inside of the capsid.
Reproductive Cycle of FMD virus in a Host Cell
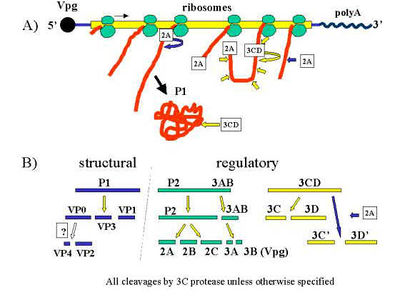
Virus replication occurs mostly in tongue and mouth cells [5]. After the virus attaches to its host cell, it enters by endocytosis and its coating from around the genome is removed [6]. The exact receptors for FMDV are not quite known, but beta integrins that are used for adhering to other cells and the cell matrix as well as interacting with extracellular proteins to perform physiological processes have been identified as FMD receptors; a receptor known as a heparan sulfate, a glycosaminoglycan, also shows affinity for FMDV [6].
After the viral RNA is in the cytoplasm of the cell, ribosomes are recruited to make negative-strand RNA to serve as viral templates. Much like other picornaviruses, the virus 'hijacks' the cell and redirects versicles to form reproduction sites within the cell. Vesicles redirected from the endoplasmic reticulum are housing the process where new viral RNA is being made, while ribosomes are synthesizing P1-encoded proteins.
Once the capsids are ready, their proteins are quickly rearranged so that the positive-strand RNA can be inserted [7]. From there, the virions burst out of the cell and travel all around the body, or if they were being produced in a blister, the fluid released by the blister exposes the virions to the air, feed, and other animals.
Viral Ecology & Pathology

Infected animals can carry the virus prior to showing symptoms and for up to four years afterwards [1]. The virus persists on solid materials and spreads rapidly through the air and when animals come into contact with each other. FMD virus can infect animals anywhere, from being transported in a contaminated vehicle, to eating feed that infected animals have also eaten from (as well as drinking from a common water source), to being inseminated by an infected animal [3].
The virus spread when cutaneous blisters, known as vesicles, rupture and fluid containing virions is released. The vesicles form in the mouth, on the lips, on hooves, and udders. Vesicles cause a range of complications for the animal: sores on the mouth make it painful to eat, ones growing on hooves cause lameness, and hooves may be sloughed off. In younger animals, heart failure is common, but adult animals do not usually die from FMD. Once an animal has recovered from the disease, it has immunity–but only to that strain [1]. Once an animal has recovered, any weight lost may not be regained, and in the case of dairy cows, milk production will always remain low [4].
Though North America has been free from any FMD outbreaks since 1929 [3], Africa, South America, Asia, and parts of the Middle East still report FMD outbreaks while most of Europe, Central and South America, Australia, and others are considered free of the disease; the UK reported an outbreak in 2007 that caused the slaughter of many animals [4]. Because vesicles may be hard to spot, especially after bursting, infections may go unnoticed, or perhaps be mistaken for other similar herd infections. Cattle, sheep, and pigs show hoof symptoms, but mouth vesicles are most common in cattle and very rare in sheep [4].
References
[1] The Center for Food Security & Public Health. "Foot and Mouth Disease." Iowa State University College of Veterinary Medicine. 2007. [6]
[2] Jackson, A.L. O'Neill H., Maree F., et al. "Mosaic structure of foot-and-mouth disease virus genomes." Journal of General Virology. 88 (2007). 487-492. [7]
[3] APHIS Veterinary Services Factsheet. "Foot-and-Mouth Disease." United States Department of Agriculture. 2007. [8]
[4] Department for Environment Food and Rural Affairs. "Foot and mouth." DEFRA, UK. 2011. [9]
[5] Horsington, J., Zhang, Z. "Analysis of foot-and-mouth disease virus replication using strand-specific quantitative RT-PCR." Journal of Virological Methods. 144 (2007). 149-155. [10]
[6] Ruiz-Saenz, J., Goez Y., Tabares W., Lopez-Herrera, A. "Cellular Receptors for Foot-and-Mouth Disease Virus." Intervirology. 52 (2009). 201-212. [11]
[7] Rybicki, Ed. "RNA Plant and Animal Virus Replication." 2009. [12]
Page authored for BIOL 375 Virology, September 2012
