Gut Microbiota in relation to Colorectal Cancer
Introduction
By Yingqi Li
Scientists believed that bodies’ biological functions are carried out by not only human genes but also the genes of the microorganisms in our bodies. The bacteria and viruses we carry serve as a buffer and interpreter of our environment. Gut microbiota plays an important role in immunological, metabolic, and neurological diseases. However, the mechanisms remain unknown. The gut bacteria supply nutrients, help with digestion, and fight against foreign opportunistic pathogens. Their behaviors are determined by the immune cells circulate throughout the body and the gut microbiota environment. It is hard to distinguish the good bacteria from the bad ones since normal gut bacteria can also trigger diseases. The over representation of one kind can lead to imbalance which induce inflammation and trigger disease in the body. [11]
Recent study has shown correlation between the composition of microbiota and human colorectal cancer (CRC). Differences in gut microbiota structural patter in colorectal cancer (CRC) patients compared with healthy people were reported. Dietary factor and dysbiosis has also been link to the risk of CRC through affecting the composition of the gut microbiota. Thus it is important for scientists to research on this interrelationship which could lead to a new way of disease prevention.[5]
Colorectal Cancer
Overview
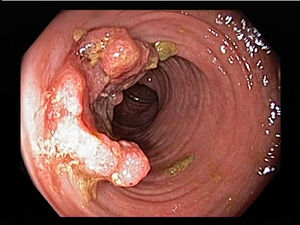
Colorectal carcinoma is one of the most common malignant tumors and most common causes of cancer-related death in the world. According to the report of WHO, colorectal cancer is the third common malignant tumor in men and second in women. Approximately 6% of the population will develop colorectal cancer during their lifetime. It was reported that in the majority of patients with colorectal cancer (about 40% to 70%), tumor cells will eventually transfer to remote organ, most of liver metastasis and then lung as second during the natural course of the disease. Liver metastases occupy 25% of patients at the time of diagnosis. About 50% of patients will develop metastasis even after operation, especially in late stage of patients. Dietary and environmental factor play a leading role in colorectal cancer. Especially in western countries where the diet is high in fat and low in fibers, colorectal cancer are more common. Another important factor is genetic alteration which can be inherited or acquired. Colorectal cancer can be a result of combined effect of multiple genetic mutations. Mechanisms include alteration of colonic flora, altered gastrointestinal tract transitional time and modulation of bile acid recycling could lead to the excess exposure to potential carcinogens within the lumen to the colonic mucosa. Recent observation in mosaic animals showed that mucosa in colon is polyclonal in its composition and each clonal progeny contains cells from a single stem cell. And the daughter cells will not extend beyond the region of confluence at the mucosal surface. Colorectal cancer formation arises from clonal expansion of a single progenitor.[12]
Treatment
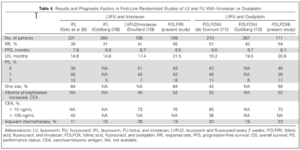
For patients with metastatic colorectal cancer, the long-term prognosis is very poor with no active treatment. So that, the operation is very important for colorectal patients. The surgery could only be done in the relatively early stage of patients (stage I-III). We have to diagnose the patients as early as possible. Screen system thus is an effective process for early diagnosis. In developed country, the process is supported by the government. But it is not easy to apply it in the developing countries. The average overall survival is only a few months in late stage of patients if not treated. Therefore, it’s rather important to choose the appropriate treatment strategies to improve the survival time.
Review of large sample studies and randomized controlled studies have shown that the R0 (without remaining tumor) resection provide the patients the chance of the long-term survival. Even in some metastatic patients (with limited metastasis), the complete resection is recommended to be performed. Even though surgical resection remains the mainstay of potentially curative therapy, the role of systemic chemotherapy has been gradually recognized for prolongation of life time in very late stage of colorectal cancer patients. [13]
Standard chemotherapy agents include 5-fluorouracil, Oxalipaltin and Irinotican. The regimens for late stage of colorectal cancer comprising 5-fluorouracil (5-FU) plus leucovorin (LV) in combination with irinotecan (FOLFIRI) or oxaliplatin (FOLFOX). It has been reported that the chemotherapy could provide 20-22 monthes of survival time. Moreover, mounting evidence suggests that the additional targeted agents (Bevacizumab and Cetuximab) might be even more effective [8]. In treating colorectal cancer, three most important strategies include surgery, chemotherapy and targeted therapy. The combination of chemotherapeutic agents and targeted agents ensured the survival time nearly 3 years. Even in late stage of patients, chemotherapy and the emergence of molecular targeted drugs can effectively control the tumor and the shrinkage of the tumor make the cancer operable. The improved complete resection will definitely provide the opportunity of survival to the patients. It was a big step for human beings in treating colorectal cancer in the past several decades. [14]
Gut Microbiota
Basic concepts of gut microbiota
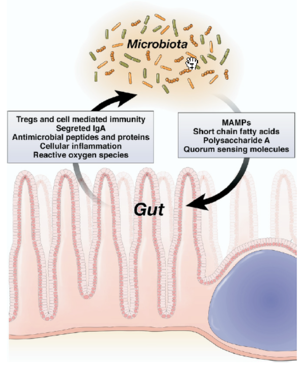
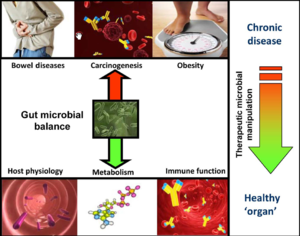
Human microbiota is great in size in both quantitative mass and qualitative diversity. Human contains a huge number of bacteria that are metabolical. The location of the bacteria is very stable, however, they vary greatly in numbers. In bacterial community anaerobe are more abundant than aerobes. The gut microbiota is typically dominated by bacteria and specifically by members of the divisions: Bacteroidetes and Firmicutes. Recently, eukaryotic fungal species have been identifies as a member of the human microbiota as well. Microbial composition start to develop since birth and varies in different individual. Altered configuration of microbiota has been associated with a number of disorders such as obesity and cancer. It was believed to be more a consequence of the disorder rather than causation. However, the correlation between the change of microbiome composition and diseases is under investigation.[11]
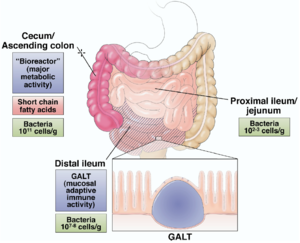
Understanding the basics about the gastrointestinal physiology and function is important for us to understand the gut microbiome. The GI track is a continuous tube that begins at the mouth and end at the anus acting as first defense of pathogens. The small and the large intestines are held in place by the mesentery. The intestines contain a barrier of mucous and epithelial cells that prevent the translocation of the bacteria in the lumen to other site in the body. The connective tissue below the intestinal epithelia called lamina propria (LP) contains a large number of intestinal immune cell. A specialized region in small intestine called Peyer’s patches is where naïve immune cells differentiate into various mature immune cells. The physical barrier and the immune cells give response when the pathogen invade though the gut. The mucus layer is the front line of the physical barrier. This layer contains glycoprotein and mucin which creates a very viscous mucus. In the epithelial layer, the tight junction protein complexes adhere the cell to each other create the second barrier. The complexes contains transmembrane, scaffold and adapting proteins. These complexes form a paracellular seal and perform as a selectively permeable barrier.[11]
Gut microbe interaction through metabolic modeling
Microbes have great metabolic capacities such as vitamin synthesis, bile salt metabolism and xenobiotic degradation. Researchers use metabolonic approach to identify different gut microbial population and their consequence metabolic process. Researches on germ free mice showed less body fat tissue than regular mice suggest the role of microbiota in energy homeostasis. Observation of reduction in bacteroidetes and increase in firmicates in obese human suggested that the microbiota of an obese person extract more energy from the diet. Microbial population changes has also been linked to other metabolic conditions such as non-insulin-dependent diabetes, non-alcoholic hepatosteatosis, and atherosclerosis. [11]
Normal microbiota exist in the mostly anaerobic luminal environment the gut microbiota generate energy through fermentation of complex carbohydrates and thus producing organic acid including short chain fatty acids (SCFAs) which are important energy source for colonic epithelium. Firmicutes such as clostridium species and bifidobacterium species are more efficient at producing SCFAs compare to other species in gut. They are also found more abundant in obese mice and human again supporting the correlation between microbial composition change and human phenotype. SCFAs serves not only as energy source but also have immunomodulatory effects such as suppressing inflammatory cytokine secretion in mice. For example, butyrate induce epithelial production of reactive oxygen species (ROS) and plays a role in NF-kB suppression. NF-kB is short for nuclear factor kB, a protein complex controls DNA transcription, cytokine production and cell survival. It plays a big role in inflammatory and immune system. It is involved in cellular response to stimuli such as cytokine, free radical and bacterial antigen. Malfunction of this protein has shown to be linked to cancer and other immune disease. Addition to suppression of NF-kB, luminal instillation of butyrate has shown promising result in human ulcerative colitis and other related inflammatory disorder. Much more research need to be done in the metabolic role of microbiota in order to understand the relationship of composition change and GI disorder thus purposing more therapeutic strategies.[11]
Immunomodulation of the gut microbiota
[[Image:Immune gut 1.png|thumb|300px|right| Hammer, A. (n.d.). The First Line of Defense: The Effects of Alcohol on Post-Burn Intestinal Barrier, Immune Cells, and Microbiome. Alcohol Research: Current Reviews, Vol. 37(No. 2), E1-E14.
In understanding immunomodulation of the gut microbiota, germ free animals have contributed a lot in researches in this field. Germ free animals are used for probiotic researches where these animals have no microorganisms living in or in them. It is known that the germ free mice have abnormal immune cell type and immune cell products. They also have a reduced level of secreted immunoglobulin and an irregular cytokine level. These observations suggested the key role of gut microbiota in the development of immune system. This is not surprising considering that intestinal mucosa has the largest surface area that is in contact with the antigens of the external environment. Also most of the antigens that are presented to the resident immune cells as well as stimulating the pattern recognition receptors like toll like receptor (TLR) and nod like receptors (NLR) of the intestinal epithelial cell lie in gut microbiota. The intestinal mucosal is composed of gut-associated lymphoid tissue (GALT), including PP and small intestine lymphoid tissue (SILT) in the small intestine, lymphoid aggregate in the large intestine, and diffusely spread immune cell in the lamina propria of the GIT. The mucosal immune system needs to fulfill the seemingly contradict functions. It need to tolerant the microbiota to prevent overt immune response as well as control the over growth and the translocation of the gut microbiota. Effects on inflammatory signaling: the epithelia can suppress TLR signaling or limit TLR expression to prevent induction of overt inflammation. [15]
Members of microbiota including nonpathogenic prokaryotes, intestinal symbiont B thetaiotamicron and symbiotic bacterial in general influence signaling intensity. Nonpathogenic prokaryotes are able to suppress inflammatory signaling pathways mediated by either intact viable organisms or secreted products. B thetaiotamicron influences the inflammatory signaling by inhibiting NF-kB pathway through the regulation of translocation of the P65 unit which is key to proliferation of cells. Symbiotic bacteria influence inflammatory pathway by manipulating the ubiquitin system. The ubiquitin system is involved in the immune response, development and programmed cell death. The targeted cell is ligated to the ubiquitin during the process of degradation. An example of symbiotic bacteria influence is the blocking of IkB, inhibitory component of the NF-kB pathway, ubiquitination which regulate NF-kB activation. As human disease is significantly related to the immune system, the high impact of the gut microbiota suggesting a strong correlation between gut microbiota composition and progression of disease such as cancer. [15]
Regulation of adaptive immunity
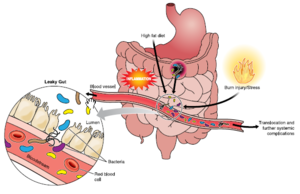
Adaptive immunity is referred to an immune response specific to antigens. This the immune response in more complex and more specific than the innate response. Factors of the level of the adaptive immune response include the ecological fitness antigenic degeneration and pathogeniticity. The gut microbiota plays an important role in the mucosal adaptive immune system.
The major components of the system is mainly found in the small intestines and are strongly influenced by microbiota. The collective gut-associate lymphoid tissue (GALT) structured as Peyer’s patch is the largest immune organ in the human body. The Peyer’s patch is mucosal lymph nodes covered with M cells, a cell type that engulf specific antigens from the gut lumen. As the normal microbiota is constantly sampled by the M cells, the mucosal immune system developed tolerance to these gut residents. Other than the M cells, the dendritic cells and the regulatory CD4+ T-cell population (Treg) are also involved in this process. Treg is also known as suppressor T-cells that helps maintain tolerance to self-antigens. Treg works through the elaboration of cytokine that suppress effector lymphocytes, including interleukin (IL)-10, transforming growth factor B etc. This distinctive characteristic of the adaptive immune system regulates the response towards antigens which abrogates autoimmune diseases. And importantly, these processes are restricted to GALT and vicinal mesenteric lymph nodes. [2]
In study of germ free animals, the introduction of a representation member of the normal microbiota bateroids fragilis induced the remediation of the undeveloped GALT, reduced CD4 T-cell population and the unbalanced Th cell subsets which indicate the importance of gut microbiota in development of the mucosal adaptive immune system. As oppose to the cell-mediated immunity, mucosal adaptive immune response also undergoes another mechanism, the humoral immunity, through secretory immunoglobulin A (IgA). Secretion of IgA is also restricted to the confines of the mucosal GALT, again illustrate the importance of gut microbiota in immune system. [11]
Relationship between Gut Microbiota and Colorectal Cancer
Inflammation, microbita and CRC
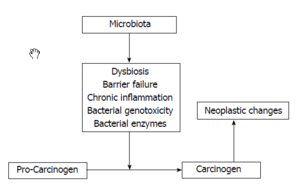
The relationship between inflammation and cancer has attracted the attention of the researchers in recent years. Cancer associated with bacterial infectious agents is found in 20% of all malignancies. Inflammations can alter the local immune response by affecting the mediators such as IL-1, tumor-necrosis factor and IL-8. This association between inflammation and cancer has been highlighted in the studies of inflammatory bowel disease (IBD) as there is an increasing evidence showing high risk of CRC in patients with IBD through the alteration of the gut microbiota.[5]
Chronic inflammation is characterized by infiltration of damaged tissue. It is known that IBD patient are prone to develop IBD associated cancer. However, research showed similar mechanism and between IBD associated and sporadic cancer. (4)Moreover, Gao’s group reported an enrichment of Fusobacterium within the tumor microenvironment. It is known that Fusobacterium may be associated with IBD including the ulcerative colitis and Crohn’s disease and these two diseases are known risk factor of colorectal cancer. Therefore, it is reasonable to hypothesis that IBD may give rise to sporadic cancer though changing the gut microbiota.[4]
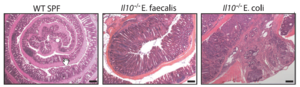
Arthur et al. introduced the two-hit model of inflammation action where inflammation creates microbiota consisting of abundant bacteria populations that synthesize genotoxins. One genotoxin called pks, synthesized by E. coli, leads to DNA damage and tumorigenesis in mice lacking the gene for IL10 (an anti-inflammatory cytokine) and treated with AOM (colon-specific carcinogen). Their experiments showed that pks by itself has the ability to cause DNA damage, and the E.coli abundance in mice IL10-/- mice increases pks production and causes further damage to DNA. The presence of both pks and AOM leads to tumorigenesis. In the second part of the model, inflammation enhances the ability of genotoxin-producing bacteria to attach to the colonal mucosa by disrupting the natural protective process that exists in WT mice. Based on their result, Arthur’s group concluded that inflammation promotes carcinogenesis by modifying the host physiology and the microbiota.[10]
Cipe’s group performed an experiment on rat in order to find out the effect of the intestinal microbiota on bioactive carcinogenic compounds. This study demonstrated the enzyme activity of B-glucosidase on formation of methylazoximethanol. Azoxymethane (AOM), known as a colon carcinogen, was first hydrolyzed to methylazoximethanol in the liver and later converted in to methyl carbon ion by B-glucosidase. Lactobacillales such as L. Casei and L. Acidophilus has shown to have anti-carcinogenic effect by suppressing the microbial enzyme activities. In intestinal hemostasis, a proposed mechanism of the protective role of SCFA is that SCFA, especially butyrate, induce apoptosis in colorectal cancer through inhibition of histone deacetylase and activation of mitochondrial apoptosis. Study showed that Helicobacter hepaticus and Bacteroides fragilis are wide spread intestinal bacteria that induce the development of colon tumorigenesis.[6]

Microbiota disbiosis and CRC
Recently, the correlation between dysbiosis and colorectal cancer (CRC) has gain a lot of interest in the research field. Studies suggested a difference of structural segregation between the cancer patients and healthy people is related to CRC, however, it is not yet clear whether the over-representation or under-representation of particular microbial species is indicative of a contributory role in the development of CRC.
Gao’s group performed a pyrosequencing based analysis of the 16S rRNA gene V3 region to investigate the difference between the cancerous tissue and the normal tissue in CRC patients. A significant over presentation of Firmicutes and Fusobacteria and relative under representation of Proteobacteria were observed in cancerous tissues. Firmicutes is associate with energy resorption indicate metabolic change in gut mocrobiota in CRC patient. An overrepresentation of Shigella was observed. This specie is an agent involved in human diarrheal disease that could increase individual’s susceptibility to CRC. In addition, certain Escherichia coli strain present in the polyketide synthetase (pks) can induce single strand DNA beaks which can be associated to the early stage of CRC. [2]
Sohani’s group pyrosequenced the bacterial gene of a random sample of CRC patient and healthy patient in order to establish the cancer-related dysbiosis. The test revealed a significant elevation of the Bacteroides/Prevotella population in cancer patients that appeared to be linked with elevated IL7. However, the association between the elevation and malignant colon cancer is not clear. Also they found in their study that the bacterial dysbiosis is not dependent on age.[3]
Marcheis’s group compare the microbes colonizing the cancerous tissue and the healthy tissue by rRNA sequencing in order to investigate the association between colonic dysbiosis and human CRC. Surprisingly, no consistent over representation of potential pathogenic bacteria in CRC tissue were found. However, the overrepresented species including Coriobacteridae, Roseburia, Fusobacterium,and Faecalibaterium, which are usually regarded as gut commensals suggesting dramatic change in physiology and metabolism.[7]
Diet, micorbiota and CRC
Dietary changes in particular have been shown to have significant effects on the microbiota. For example, Japanese people have a unique enzyme acquired from a marine bacterium to help digest seeweed that is common in their diet. On the other hand, African people are enriched in Bacteroidetes and lack of Firmicute due to their high fiber containing diet. It has been shown in mice that change in in microbiota composition happened in a day after switching to a high-fat, high-sugar “western” style from a low fat, high-fiber diet. The correlation between the gut microbiota composition and gastrointestinal cancer was seriously examined. Research showed that huge impact of dietary factor on composition of gut microbiota can actually influence human health and disease.[1]
The molecular interaction of gut microbiota is greatly influenced by diet. It is reported that the production of butyrate, folate, propionate and biotin from digestion lead to epigenetic modification such as changes in DNA methylation and histone acetylation or more indirectly inhibit certain enzymes.[1]
Conclusion
One limitation of the study on the correlation between the CRC and the gut microbiota is that the sample size is fairly small. Further study on wider sample sizes will be needed to determine the role of certain gut microbiota. Additional interest towards researching microbiota includes its potential value as a marker for colon cancer.
This glimpse of the CRC microbiome provides an important step towards full understanding of the relationship between intestinal microbial ecology and CRC, which may help leading us towards novel CRC diagnostic tools and therapeutic intervention.
References
[1]
1.Clemente, J., Ursell, L., Parfrey, L., & Knight, R. (n.d.). The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell, 1258-1270
2.Gao, Z., Guo, B., Gao, R., Zhu, Q., & Qin, H. (2015). Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. Frontiers in Microbiology.
3.Sobhani, I., Tap, J., Roudot-Thoraval, F., Roperch, J., Letulle, S., Langella, P., . . . Furet, J. (2011). Microbial Dysbiosis in Colorectal Cancer (CRC) Patients. PLoS ONE.
4. Hope, M., Hold, G., Kain, R., & El-Omar, E. (n.d.). Sporadic colorectal cancer â role of the commensal microbiota. FEMS Microbiology Letters, 1-7.
5.Tlaskalova-Hogenova, H. (2011). The role of gut microbiota(commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune disease and cancer: Contribution of germ free and gnotobiotic animal models of human disease. Cellular & Molecular Immunology, 8, 110-120. doi:10.138/cmi.2010.67
6.Cipe, G. (2015). Relationship between intestinal microbiota and colorectal cancer.World Journal of Gastrointestinal Oncology, 7(10), 233-240. doi:10.4251/wjgo.v7.i10.233
7.Marchesi, J., Dutilh, B., Hall, N., Peters, W., Roelofs, R., Boleij, A., & Tjalsma, H. (2011). Towards the Human Colorectal Cancer Microbiome. PLoS ONE.
8.Chen, W., Liu, F., Ling, Z., Tong, X., & Xiang, C. (2012). Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE.
9.Wang, T., Cai, G., Qiu, Y., Fei, N., Zhang, M., Pang, X., . . . Zhao, L. (2011). Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. The ISME Journal ISME J, 320-329.
10.Arthur, J., Perez-Chanona, E., Muhlbauer, M., Tomkovich, S., Uronis, J., Fan, T., . . . Jobin, C. (2012). Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science, 120-123.
11.Neish, A. (2009). Reviews in basic and clinical gastroenterology. Gastroenterology,136, 65-80. doi:10.1053/j.gastro.2008.10.080
12.Hurwitz, H. (2005). New Combinations in Metastatic Colorectal Cancer: What Are Our Expectations? The Oncologist, 320-322.
13.Tournigand, C. (2003). FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study. Journal of Clinical Oncology, 229-237.
14.Goldberg, R., Rothenberg, M., Cutsem, E., Benson, A., Blanke, C., Diasio, R., . . . Viele, C. (2007). The Continuum of Care: A Paradigm for the Management of Metastatic Colorectal Cancer. The Oncologist, 38-50.
15.Kato, L., Kawamoto, S., Maruya, M., & Fagarasan, S. (2014). The role of the adaptive immune system in regulation of gut microbiota. Immunol Rev Immunological Reviews, 67-75.
Authored for BIOL 291.00 Health Service and Biomedical Analysis, taught by Joan Slonczewski, 2016, Kenyon College.