Koala Retrovirus
A Viral Biorealm page on the family Koala Retrovirus

Koala Retrovirus (KoRV), the causative agent of Koala Immunodeficiency Syndrome, is a retrovirus that infects Koalas (Phascolarctos cinereus), especially in populations located in mainland Australia. Koala retrovirus is believed to be a primarily endogenous virus present in a large proportion of Australian koala populations. Studies have suggested that the virus is responsible for the very high incidence of chlamydosis, leukemia, and lymphoma seen in koalas [1,2,7,12]. The primary method of viral proliferation is vertical transfer via an endogenous form of the virus, while evidence exists that the virus is transmitted horizontally as a membrane-enclosed virion only through close contact between individuals [2,7,12]. KoRV associated disease provides a significant risk to koala populations in the wild and in captivity, and when combined with factors such as habitat destruction, raises questions as to the conservation status of the species.
Virus Classification and Higher Order Categories
Baltimore Classification: Group VI (+ssRNA)
Family: Retroviridae
Genus: Gammaretrovirus
The koala immunodeficiency virus is a retrovirus falling into the group VI division of the Baltimore Classification system. Koala retrovirus virions contain positive-sense single stranded RNA, and have a DNA intermediate in their replication cycle (+ ssRNA-RT). Koala retrovirus belongs to the family Retroviridae, and the genus Gammaretrovirus. Particular species of the virus are yet to be identified.
Description and Significance
Koala immunodeficiency virus is perhaps the most significant health concern facing koalas today. Infection with KoRV has been shown to be linked to increased prevalence of leukemia, lymphoma, and chlamydia [1,3]. Studies have shown increased blood plasma viral RNA levels in both leukemic and chlamydia positive Koalas [1]. The virus has a 100% prevalence in the blood and various tissues of koalas living in Southeast Queensland Australia [1,2]. Though the virus is present in 100% of this mainland population, not all virus-carrying koalas express symptoms of the disease [2].

The virus exists primarily in an endogenous form [1,2,3,4]. However, KoRV virions have been observed in infected tissues of lymphoma and chlamydia positive koalas [1]. This virus is particularly significant as the dynamics of how the virus is spread are yet to be fully understood. While it appears that most of the viral proliferation occurs vertically with host replication, virions are still produced at times [1,2]. This suggests that from an evolutionary standpoint, the virus is at a transition between a completely endogenous virus, and a virion-based pathogen. It appears that this evolution of this dynamic is fairly recent, as the isolated (since 1920) koala population on Kangaroo island, showed no incidence of the virus until very recently [2]. Recent transmission of the virus, possibly by mosquitoes, has reduced the koala population on Kangaroo Island by half [5]. Thus, evidence exists that the retrovirus may still be transferred horizontally and that the retrovirus is yet to be completely endogenized. Further evidence is provided for this conclusion by the discovery that the copy number, sequence, and position in the genome of the KoRV provirus varied greatly between sampled koalas [7]. This ongoing endogenization of KoRV into the koala genome provides a rare, observable model of how viral genetic material (such as those that make up a significant portion of mammalian genomes) is permanently made part of the host genome [6,7,1].
Beyond the significance and importance of the mechanisms of evolution and proliferation of KoRV, rising public awareness of the virus has also started a key debate as to the conservation status of Koalas. Though the koala population size remains relatively high, some surveys have noted that the koala population in Australia has dropped from 100,000 to 40,000 over 6 years [8]. This alarming decline (which is also due in part to urbanization, car accidents) coupled with the fact that there is no successful treatment or vaccine for KoRV has led to the conclusion that koalas may be extinct in 30 years [8,9]. There is an ongoing debate in Australia as to what to do about the virus. While Koalas are particularly important in the tourism sector in eastern Australia [8], some groups resent their voracious feeding habits, while others few any intervention as an interruption of natural selection [10]. The amount of funding allocated to research aimed at understanding KoRV has also been criticized as "pathetic" by members of the Australain veterinary scientific community [5].
However, research beyond Australia has been on the rise, particurally due to the rising incidence and effect of KoRV in captive populations abroad [11]. For example, studies at the Koch Institute in Berlin, Germany utilized human kidney-293 cells and rat models to confirm that KoRV can replicate and infect via an exogenous (horizontal transmission) mechanism [12]. These researchers also elucidated a link between KoRV infection and a host immune response, showing that as viral load is increased in human kidney cells, secretion of anti-lymphoproliferative cytokines increases[12]. This study also demonstrated that KoRV is capable of replicating in certain human cells, and is capable of productive (though unsustainable) infection of rats [12]. This key finding highlights the significant ability of KoRV to perform transpecies infection, though it is important to note that it was not observed to cause immunosuppression in non-koala hosts. Due to the existing high rate of KoRV infection (particularly as a provirus) in koalas, the ability of the virus to be transferred to uninfected populations horizontally, and the association of the virus with lethal illness, the conservation status of koalas in the wild and in captivity remains very much in question.
Genome Structure
Koala Retrovirus has a positive sense single-stranded RNA genome of 8,431 nucleotides in length [1,3]. Studies have indicated that that the virus showed a high degree of homology (78% nucleotide sequence similarity) with the Gibbon Ape Leukemia Virus(GALV), as well as other simian type-C retroviruses[1,3]. In fact the degree of similarity between these two viruses is so high that they are hypothesized to have only recently diverged on an evolutionary scale [3,7,9]. In line with other type C gammaretroviruses, the KoRV genome contains LTR, gag, pol, and env regions [3]. In addition to three open reading frames corresponding to the gag/pol/env sequences, while several small ORFs were also identified, but have unknown function [3]. The gag and pol RNA sequences of KoRV are particularly similar to those of GALV, with similarity percentages of 82% in both cases [3]. In a retrovirus such as KoRV,when viral RNA enters a host cell it is first converted to DNA by a packaged RT protein packaged in the virion. The pol gene found in the genome of KoRV encodes this essential protein needed for horizontal transmission [13]. Similarly, the env gene encodes envelope peplomer proteins, and the gag gene encodes the virion capsid proteins [13].
Virion Structure of a Koala Retrovirus
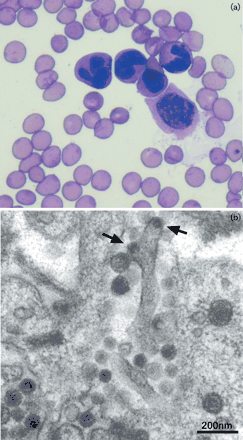

The structure of Koala Retrovirus virion is not yet well understood. As a primarily endogenous virus that proliferates vertically as an integrated part of its hosts genome, KoRV virions are more difficult to observe. However, recent studies utilizing thin-section electron microscopy have observed the presence of small virion particles budding off of infected bone marrow cells in koalas suffering from leukemia [1]. Other studies utilizing electron microscopy have characterized KoRV virions as membrane enveloped particles with a dense core, an observation which is analogous to observations of other type C retrovirus virions [11]. Type C-gammaretrovirus virions are typically about 80-100 nm in diameter, with an outer membrane derived from the membrane of a host cell [13]. Within this host-derived membrane is an icosohedral capsid containing a reverse dimer of the viral (+) ssRNA as well as vital proteins such as viral reverse transcriptase [13]. Gammaretrovirus virions also present glycoprotein peplomers outward from the outer host-derived membrane [13]. In contrast to other retroviruses, type C gammaretrovirus particles begin formation when nascent C-shaped nucleocapsids align near the host cell membrane [13]. Koala retrovirus virions, which appear to closely resemble the structure of other type-C gammaretroviruses, likely remain an important means of horizontal transmission, though the permanent endogenization process of the virus appears to be progressing.
Reproductive Cycle of a Koala Retrovirus in a Host Cell
As discussed above, KoRV primarily proliferates as a provirus through a vertical transmission mechanism (from parent to offspring). However, the ability of KoRV virions to proliferate and infect additional target cells has been observed (see above). In most cases, the KoRV provirus is replicated as part of its host's genome[1,2,3,9]. This means that the virus is continually passed on to essentially every koala born in eastern Australia, where virtually all koalas are virus-positive [9].
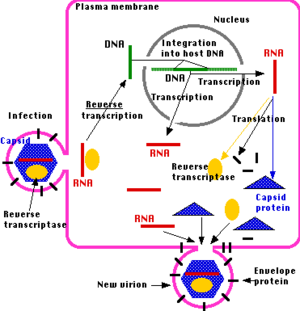
While the exact mechanism of KoRV virion production and infection of target cells is not known, the structural similarities between it and other gammaretroviruses suggest that it roughly follows the general infection mechanism of these viruses. In a typical gammaretrovirus infection cycle, membrane enclosed virions first fuse with the cell membrane of target cells, and insert the capsid containing the two copies of the RNA genome. This is achieved by the recognition of a particular cellular membrane protein by a viral protein presented on the exterior of the viral membrane [14,15]. Once the capsid enters the target cell it is degraded, releasing the viral RNA and reverse transcriptase (RT) into the cytoplasm [14,15]. Viral RT recruits nucleotides from cytoplasmic pools and uses the viral RNA as a template to synthesize a double-stranded DNA copy of the retroviral genome [14,15]. The DNA migrates through a nuclear pore and is inserted into the host genome by another viral protein, integrase [14]. This is the point of divergence for the active, multi-copy producing replication cycle and the endogenous replication cycles displayed by KoRV. Often times, the inserted KoRV provirus only proliferates as the host cell replicates.
However, virion production is still common. Following viral insertion into the host genome, transcription by the host cell produces viral mRNA. This viral mRNA can take one of three routes: (1) serve as a viral genome for virions, (2) serve as a template for translation to produce a gag-pol or gag polyprotein, (3) serve as a template for translation to produce an env polyprotein [14]. Viral proteases cleave the polyproteins into functional viral proteins [15]. Following this, gag-derived proteins bind retroviral RNA copies and assemble with other viral polyproteins to form viral particles that assemble at the cell surface [14]. Finally, the viral particles begin to bud out, creating an envelope around the viral capsid [15]. While the virions are budding out of the host cell, a protease cuts itself out of the gag-pol polyprotein and cleaves other polyproteins in the immature retroviral virion [15]. In the case of HIV, drugs have been developed to attempt to disrupt this protease to prevent production of mature virions [15]. Thus, it may be possible to disrupt the production of KoRV virions with a similar drug, though no such studies have been performed.
Viral Ecology & Pathology

Detection of KoRV in koala hosts is typically performed via PCR of sampled blood or bone marrow tissue [1,9]. The provirus that all KoRV infected koalas express is active, and releases viral RNA and occasionally virions [9]. While it has not been directly proven that KoRV actually causes leukemia, lymphoma, and chlamydia in its host, there is a very strong positive correlation between viral presence (as well as viral load) and these diseases [1,7,9,16]. Therefore, any koalas showing signs of chlamydia, lymphoma, leukemia, or other cancers, are treated by Australian wildlife services as suspects for harboring the virus [9]. The exact mechanism by which KoRV suppresses the immune system is also unknown. Due to the near universal distribution of the virus in many populations, immunosupression from KoRV likely contributed to whatever illness the koala is displaying. KoRV-associated lymphoma can typically be identified by the presence of neoplastic cell showing cleaved or donut shaped nuclei [9]. Clinical signs of KoRV associated lymphoma and leukemia include grossly enlarged peripheral lymph nodes as well as general weight loss and diarrhea [9].
While it is well understood that KoRV proliferates primarily by a vertically transferred provirus, the mechanisms surrounding horizontal virion based transport are not well understood. Koalas pass the virus to their offspring as the provirus has colonized the animal's germ cells, which give rise to gametes [2]. Close proximity is thought to be required for transfer between koalas though no definitive findings have been made [2,7]. There is some speculation that mosquitoes may be able to transfer the virus between koalas by carrying blood meals, though that has not been directly tested [5]. Additional studies have shown that ticks may be capable of transferring KoRV RNA, though it was noted that this laboratory experiment did not accurately portray typical koala-tick interactions [16]. Hence, the exact mechanism of KoRV transmission between koala hosts remains an open question, though insect intermediates may play a role. Unfortunately, no vaccine or reliable treatment exists for KoRV or the associated cancers. These associated cancers account for up to 60% of koala mortalities in captive areas and at least 5% in the wild [3].
References
[1] Tarlinton R, Meers J, Hanger J, Young P. 2005. Real-time reverse transcriptase PCR for the endogenous koala retrovirus reveals an association between plasma viral load and neoplastic disease in koalas. Journal of General Virology. 86: 783-787.
[2] Tarlinton R, Meers J, Young P. 2006. Retroviral invasion of the koala genome. Nature. 442: 79-81.
[3] Hanger JJ, Broham LD, McKee JJ, O'Brien TM, Robinson WF. 2000. The nucleotide sequence of Koala Retrovirus: a Novel Type C Endogenous Virus Related to Gibbon Ape Leukemia Virus. Journal of Virology. 74(9): 4264-4272.
[4] Oliveira NM, Farrell KB, Eiden MV. 2006. In Vitro Characterization of a Koala Retrovirus. Journal of Virology. 80(6): 3104-3107.
[5] Hockley C. 2011. "Retrovirus threatens Kangaroo Island koalas." adelaide news. http://www.adelaidenow.com.au/retrovirus-threatens-ki-koalas/story-e6frea6u-1226149865026. Accessed: 9/20/12.
[6] Gifford R and Tristem, M. 2003. The evolution, distribution, and diversity of endogenous retroviruses. Virus Genes 26: 291-315.
[7] Tarlinton R, Meers J, Young P. 2008. Biology and evolution of the endogenous koala retrovirus. Cell. Mol. Life Sci. 65: 3413-3421.
[8] "Koala Aids Spreading." CNN video. http://wn.com/koala_aids_spreading. Accessed on: 9/20/12.
[9] Tarlinton R. 2005. "Koala Retrovirus." http://ebookbrowse.com/koala-retrovirus-korv-19-jul-2005-1-0-pdf-d294848280. Accessed: 9/21/12.
[10] Racaniello V. 2009. "A retrovirus is invading the Koala genome." http://www.virology.ws/2009/04/21/a-retrovirus-is-invading-the-koala-genome/. accessed: 9/19/12.
[11] Worley M, Rideout B, Shima A, Janssen D. 1993. Oppurtunistic infections, cancer and hematologic disorders associated with retrovirus infection in the koala (Phascolarctos cinereus), 181-182. In: Proceedings of the American Association of Zoo Veterinarians, Saint Louis, Mo.
[12] Feibig U, Hartmann MG, Bannert N, Kurth R, Denner J. 2006. Transpecieis transmission of the endogenous koala retrovirus. Journal of Virology. 80: 5651-5614.
[13] Murphy FA, Gibbs EPJ, Herzinck MC, Studdert MJ. 1999. Veterinary Virology. Elseveir. pg 364-372.
[14] "Retrovirus Life Cycle Tutorial." Nolan Lab, Stanford University. http://www.stanford.edu/group/nolan/tutorials/ret_2_lifecyc.html. Accessed:9/22/12.
[15]"HIV Replication Animation." W.W. Norton. http://www.wwnorton.com/college/biology/microbiology2/ch/11/animations.aspx. Accessed: 9/22/12.
[16]Simmons G. 2011. The Epidemiology and Pathogenesis of Koala Retrovirus. PhD Thesis, School of Veterinary Science, The University of Queensland. http://espace.library.uq.edu.au/view/UQ:244963. Accessed: 9/23/12.
Page authored for BIOL 375 Virology, September 2012
