Methanogenic Anaerobic Digestion of Wastewater

In the present day and age, there is a large emphasis on green technology and environmental sustainability. For sustainability to be achieved, the amount of pollution generated must be reduced, and society must become less dependent on fossil fuels as its main source of energy.1 These two goals can be achieved concurrently via the treatment of wastewater. Wastewater contains high concentrations of degradable organic material which, when processed, produce valuable bioenergy or biochemicals.1 Wastewater treatment methods vary, but include methanogenic anaerobic digestion, biological hydrogen production, microbial fuel cells, and fermentation for production of valuable products.1
This page focuses on methanogenic anaerobic digestion of wastewater.
Types of Methanogenic Anaerobic Digestion
Digestion of organic matter in wastewater by methanogenic anaerobic methods is popular because as compared to conventional aerobic methods, it17:
- Is more simple in construction and operation
- Requires no external power
- Is compact
- Generates little biological sludge waste
- Has high treatment efficiency
- Has low capital and operating costs
- Does not require oxygen
- Generates methane fuel
Because anaerobic treatment methods do not require the input of oxygen, it is also more cost efficient, particularly in tropical environments, where methanogenic bacteria thrive.6 The organic material present in wastewater come from a variety of sources, including wastes from domestic, commercial, industrial processes. It is vital that these organic compounds are digested because if they are released into the environment, it will result in an increase in the biological oxygen demand and chemical oxygen demand, which poses a threat to aquatic organisms.9
The conversion of organic material present in wastewater to methane follows a four-step process.
- Fermentative bacteria hydrolyze complex organic polymers, such as proteins, polysaccharides, and lipids, to amino acids, sugars, and fatty acids.
- Monomers are then fermented to intermediates containing a mix of organic alcohols and acids.
- Acetogenesis further oxidizes the intermediates to acetic acid, hydrogen, and carbon dioxide via hydrogen-producing acetogenic bacteria.
- Methanogens to convert acetate into methane and carbon dioxide.1
Methane produced from anaerobic digestion is most often used as an on-site fuel source for heating or electricity production using fuel cells, making it a "net positive energy" process.1 Additionally, by converting it to methanol, methane can be used in the production of biodiesel.2
Three key technologies that utilize methanogenic anaerobic digestion are the upflow anaerobic sludge blanket reactor, anaerobic migrating blanket reactor, and anaerobic sequencing batch reactor.
Upflow Anaerobic Sludge Blanket (UASB) Reactor

The UASB reactor consists of a single vessel, where wastewater enters from the bottom and flows upward into the sludge granules, which filters out organic material, and results in the production of biogas and clarified effluent (Fig. 2).3 The sludge blanket is an aggregation of microbes that degrade the organic matter in wastewater, producing methane and carbon dioxide gases in the process. Sludge granules do not mix with the clarified effluent because their weights allow them to settle,1 and the reactor has sloped walls to deflect any material that reaches the top--this is known as a gas-solids-separation system.35 Treated water is extracted from the top of the tank.
Advantages of UASBs include low energy requirement, low operation and maintenance costs, low sludge production, resource recovery from biogas production, and high reduction of organic matter.4 It is predicted that the pay back period for UASB technology is less than 3 years.4 UASBs are able to reduce the chemical oxygen demand (COD), a measure of water quality, by 80-90%, thus demonstrating its high organic removal rates.3 Studies have found UASBs to be most effective in the treatment of wastewater from breweries, distillery, pulp and paper, and food processing industries.4
The majority of research on anaerobic digestion has been conducted using UASB reactors.17 Wastewater treatment plants utilizing UASB reactors to treat municipal wastewater have been built in many tropical and sub-tropical regions, such as India, Colombia, and Brazil, as well as temperate regions, including the United States and the Netherlands18.
Anaerobic Migrating Blanket Reactor (AMBR)

The anaerobic migrating blanket reactor (AMBR) is a continuously fed, staged reactor developed in response to the need for treatment of wastewater at small- to medium-sized industries (Fig. 3). Unlike UASBs, AMBRs do not require gas-solids-separation and feed-distribution systems.5 Instead of an upflow pattern, wastewater flows in horizontally from one end, and effluent leaves from the other end.5 In each compartment are microorganisms that interact with the substrate, thus filtering the wastewater.5 As water flows through each compartment, the substrate concentration decreases. The final compartment serves as a collecting tank that prevents biomass loss.5 The horizontal flow causes migration of the biomass from the first compartment to the last compartment. In order to prevent excess biomass buildup in the last compartment, the flow is periodically reversed--the last compartment becomes the first, and vice versa.5 AMBRs are still considered a novel wastewater treatment system. However, studies have shown that it has some advantages over UASBs, including17:
- Low biomass migration rate.
- Less chance of short-circuiting.
- Efficient removal of poorly biodegradable compounds.
- Can be used for high-strength wastewater.
However, the design of AMBRs is more complex than UASB, and thus requires gentle mixing to maintain contact between the substrate and biomass, and to prevent clogging of the microbial sludge.5
Anaerobic Sequencing Batch Reactor (ASBR)
In ASBRs, a single vessel contains microorganisms that are mixed with the wastewater. Once the biomass settles, the clarified effluent is withdrawn from the reactor.1 ASBRs have been proven to be highly effective for agricultural wastewater.1 Key distinctions between Anaerobic Sequencing Batch Reactors and Upflow Anaerobic Sludge Blanket Reactors are17:
- A feed-distribution system is not needed in ASBRs
- ASBRs do not use a three-phase separator--instead, it has a single vessel.
- No upflow hydraulic pattern.
- ASBRs are operated in discontinuous mode.
Evidence shows that the organic removal rate of AMBRs and UASBs are similar at low organic loading rates, but UASBs are able to outperform AMBRs at high organic loading rates.17 In addition, suspended solids retention rate in ASBR was 2.5 times higher than in UASBs.
Microbiology of Methane Digestion
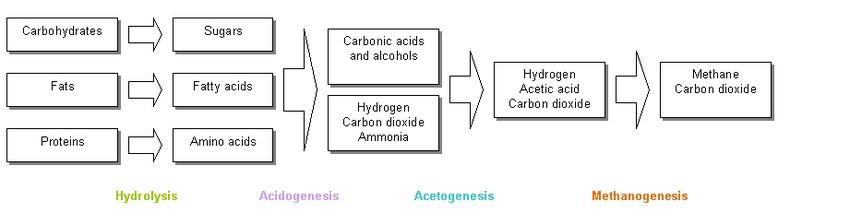
The microorganisms involved in methanogenic digestion work in synergy, where products of one reaction serves as the substrate for another group of microorganisms, thus the four-step process of converting wastewater into methane and carbon dioxide is a chain process, in which many complex biological reactions that run in parallel (Fig. 4).6 To maintain a balanced digestion process, the various conversion reactions must be sufficiently coupled during the digestion process.6 This ensures that no excess buildup of intermediates will result. 8 This is unlike aerobic processes, where only a single microorganism is needed for the degradation of wastewater. 7
Hydrolyzing and Fermenting Bacteria
Wastewater contains a large amount of organic material that needs to be broken down into monomeric units in order for microbes to be able to digest them. Suspended organic matter (SOM) must be hydrolyzed and converted to a specific size and type so they are able to pass through microbial cell walls, which will then be used as nutritional or energy sources.12 The hydrolysis of polymers is often viewed as the rate-limiting step in methane digestion because it provides the substrates, such as amino acids, glycerol, soluble sugars, and long-chain fatty acids, needed for further degradation.11 These reactions are catalyzed by enzymes secreted by a variety of bacteria, including Bacteroides, Clostridium, Fusobacterium, Selenomonas, and Streptococcus.10
Presently, the Clostridium species has been found to be one of the most active contributors to hydrolysis of organic matter.12 In a study by Kim et al. (2009), the composition of the bacterial community present during hydrolysis of SOMs was analyzed by Denaturing Gradient Gel Electrophoresis (DGGE). Results showed that Clostridium thermopalmarium, an anaerobic, moderately thermophilic species, was most dominant during hydrolysis -- present from day 1 to 8 of incubation. In addition, Clostridium sporogenes, an obligate anaerobe, and Bacillus coagulans, a facultative anaerobe, were also found to be key players in SOM hydrolysis.12
The total solid (TS) content of crop biomass, 10-50%, tends to be much higher than those of sewage sludge and liquid manure, which has a high water content. The main organic component of crop biomass is lignocellulose, which is composed of cellulose, hemicellulose, and lignin.15 Due to its heterogeneity, the hydrolysis of lignocellulose biomass requires a complex set of enzymes, secreted by cellulolytic and xylanolytic microbes.15 Though it is rare for bacteria to be able to degrade cellulose, when they are able to, they can hydrolyze plant cell walls very efficiently. Two such bacteria are Clostridium stercorarium and Clostridium thermocellum. C. stercorarium is efficient at hydrolyzing hemicellulose using its simple system of just two cellulases. C. thermocellum is one of the most efficient cellulose degrader due to its cellulosome, an extracellular enzyme complex.16 Cellulases have two cleavage mechanisms: retaining and inverting cleavage.16
Hydrogen-Producing Acetogenic Bacteria
After the organic polymers have been broken down, a group of acetogenic bacteria converts the hydrolysis products into hydrogen, carbon dioxide, and volatile organic acids (VOA).1012 These acetogenic bacteria are often the same as the bacteria involved in hydrolysis. For example, Clostridium thermopalmarium first breaks down glucose, xylose, and sucrose into soluble sugars; then uses the monosaccharides to produce H2, CO2, butyric acid, and small amounts of acetate, ethanol, and lactate as side products.12 Clostridium novyi, known to be involved with hydrolysis, also produces hydrogen and carbon dioxide from carbohydrates, such as raffinose, glucose, melibiose, and ribose.10 Kim et. al (2010) discovered that during the wastewater treatment process, the intensity of the C. novyi band corresponded to the volume of propionic acid, a VOA, accumulated. Thus, it is likely that C. novyi is the main producer of propionic acid.12
Members of the Fusibacter genus have also been found to be key players in both hydrolysis and acidogenesis. In an analysis of microbial communities in anaerobic batch reactors, Shin et al. (2010) observed that a greater intensity in the Fusibacter band on their DGGE results was in conjunction with both a decrease in concentration of volatile solids and increase in volatile fatty acids.13 In addition, Basso et al. (2009) reported that members of the genus Fusibacter are thiosulfate-reducers that produce acetate, butyric acid, CO2, and H2 from carbohydrates.14 This indicates that not only is the genus Fusibacter involved in hydrolyzing VOM to their component monomers, but it further processes the monomers into substrates that can be used in the next and final step, methanogenesis.14
Other bacteria present in acidogenic microbial communities include:14
- Dethiosulfatibacter aminovorans: a thiosulfate-reducing bacterium that produces CO2, H2, acetate, and propionate from VOM.
- Clostridium aminobutyricum: a spore-forming anaerobe that ferments amino acids, producing acetate, butyric acid, and ammonia.
- Clostridium sticklandii: utilizes the Stickland Reaction to form acetate, butyric acid, and ammonia.
- Lactobacillus delbrueckii subsp. bulgaricus: forms lactate from lactose.
Methane Forming Bacteria
Methanogens are a group of strictly anaerobic Archaea which carry out methanogenesis. The major precursor for methanogenesis is acetate (70%).14 It was previously thought that Methanosarcinales were the dominant methanogenic group, but recent studies have showed that shifts in the composition of bacterial sludge occur during different phases of methanogenesis.13 Shin et al. (2010) observed that a M. conilii-like archaeon, part of the Methanosarcinales group, was the most abundant species at day 0 of incubation.13 Between day 9.9 and 11.9, there was both a sharp increase of the M. conilii-like archaeon, as well as the appearance of a M-mazei-like archaeon, part of the Methanosaeta species.13 Scientists speculate that this community shift occurs for two reasons: because the growth rate of Methanosarcina spp. is higher than that of Methanosaeta; and because the M. conilii-like Methanosarcina has a high affinity for acetate. After the acetate is used up, the M. mazei-like methanogen, which can use non-acetate sources for conversion to methane, appears and stabilizes the microbial sludge.13
H2 is another precursor for methane formation during anaerobic digestion. However, the thermodynamic limitations of hydrogen-mediated metabolism has required that anaerobic digestion systems maintain low hydrogen partial pressure in order for microorganisms to be able to make use of intermediates produced in acidogenesis and acetogenesis.13 To achieve this, hydrogen-utilizing microorganisms, such as Methanomicrobiales, are needed in large quantities at the beginning of methanogenesis.13 M. petrolearius is one such microbe that produces methane from H2, CO2, formate, and 2-propanol. Also present in the hydrogen-mediated methanogenic population include Methanocorpusculum bavaricum-like, Methanogenium marinum-like, and Methanocalculus pumilus-like organisms, all part of the Methanomicrobiales group.13 This indicates that once acetate is used up, the microbial community shifts from being Methanosarcinales-dominant to Methanomicrobiales-dominant.13
Process Parameters
Because anaerobic digestion of wastewater is facilitated by populations of microbes, it is important to maintain certain environmental conditions in order to ensure optimal function of microorganisms.
Temperature
The microbes that convert wastewater into methane thrive under mesophilic and thermophilic conditions, so most anaerobic digestion plants are operated at 35°C.6 However, because the raw influent usually enters the digestion system at temperatures below 18°C, heat is required to raise the temperature of the wastewater. 6 In order to bring the wastewater up to temperature, the plants consume up to 30% of the energy produced by anaerobic digestion. 6 The generation time of methanogenic bacteria range from 3 days at 35C to 50 days at 10C.17 Thus, it is important for wastewater plants to operate at higher temperatures because a prolonged regeneration time lowers biochemical activity and efficiency, which ultimately results in a decrease in methane yield. 6
pH
Acid-producing bacteria have an optimal pH range of 5.0 to 6.0. However, the pH range for optimal function of methane-producing bacteria was found to be very narrow, ranging from 6.7 to 7.4.17 Because of the greater sensitivity of methanogens to pH, anaerobic wastewater treatment systems operate at a pH between 6.0 to 8.0 in order to maintain high levels of activity in methane-producing microorganisms. The lowering of pH caused by acid-producing bacteria is buffered by bicarbonate produced by methane-producing microbes.17
Nutrients
Rajeshwari et al. (2000) found that nitrogen, phosphorus, and trace elements, such as magnesium, iron, nickel, sulphur, potassium, calcium, cobalt, zinc, manganese, and copper, are required for the growth of microorganisms involved in anaerobic digestion.18 Because these elements are present in sufficient amounts in most wastewater, they do not need to be supplemented into the seed sludge.18
Some heavy metals, especially copper and zinc, have been found to have toxic effects on acidogenesis.19
Further Reading
In addition to anaerobic digestion of wastewater, advances have been made in the digestion of human wastes and food wastes to produce biogas and electricity. Anaerobic digestion is a very viable solution to environmental sustainability, as it able to reduce biomass wastes, improve sanitation, control both water and air pollution, and can act as an alternative energy source—thus reducing greenhouse gas emissions.20 In addition, the production of biogas makes it an extremely attractive option for developing nations, where energy is both costly and in short supply, as it can be used for a wide variety of daily activities, including cooking, lighting, and heating.20
References
1. Angenent L., Karim K., Al-Dahhan M., Wrenn B., Domiguez-Espinosa R. (2004). Production of bioenergy and biochemical from industrial and agricultural wastewater. Trends Biot., 22(9): 477-485.
2. Witt, P.M. and Schmidt, L.D. (1996) Effect of flow rate on the partial oxidation of methane and ethane. J catal. 163: 465-475
3. Upflow Anaerobic Sludge Blanket Reactor. Akvopedia.
4. Tare V. and Nema A. UASB technology - expectations and reality. Dept. of Env. Engg., IITT Kanpur, India
5. Angenent L.T. and Sung S. (2001). Development of anaerobic migrating blanket reactor (AMBR), a novel anaerobic treatment system. Wat. Res., 35(7):1739-1747
6. Mrowiec B. and Suschka J. Anaerobic Wastewater Treatment Process. University of Bielsko-Biala.
7. Marchaim, Uri. Biogas Processes for Sustainable Development. Rome: FAO, 1992.
8. Parawira W. (2004). Anaerobic treatment of agricultural residues and wastewater. Application of high rate reactors. Doctoral Dissertation, Department of Biotechnology, Lund University, Sweden.
9. Lee C. N. (2011) Removal efficiency and kinetic study of BOD and COD using aerobic and anerobic digestion. Bachelors' Disserataion, Universiti Tunkul Abdul Rahman.
10. Food and Agriculture Organization of the United Nations. (1998). Renewable biological systems for alternative sustainable energy production. Chapter 4.
11. Arsova, L. (2010)Anaerobic digestion of food waste: Current status, problems, and an alternative product. Columbia University Department of Earth and Environmental Engineering.
12. Kim M.D., Song M., Jo M., Shin S. G., Khim J. H., Hwang S. (2010). Growth condition and bacterial community for maximum hydrolysis of suspended organic materials in anaerobic digestion of food waste-recycling wastewater. Appl Microbiol Biotechnol., 85(5):1611-1618.
13. Shin S.G., Lee S., Lee C., Hwang K., Hwang S. (2010). Qualitative and quantitative assessment of microbial community in batch anaerobic digestion of secondary sludge. Bioresour Technol., 101(24): 9461-9470.
14. Basso O., Lascourreges J.F., Le Borgne F., Le Goff C., Magot M. (2009). Characterization by culture and molecular analysis of the microbial diversity of a deep subsurface gas storage aquifer. Res Microbiol., 160(2):107-116.
15. Cirne D.G., Lehtomäki A., Björnsson L., Blackall L.L. (2007). Hydrolysis and microbial community analyses in two-stage anaerobic digestion of energy crops. J Appl Microbiol., 103(3):516-27.
16. Zverlov V.V., Schwarz W.H. (2008). Bacterial cellulose hydrolysis in anaerobic environmental subsystems--Clostridium thermocellum and Clostridium stercorarium, thermophilic plant-fiber degraders. Ann N Y Acad Sci., 1125:298-307
17. Liu Y., Tay J.H. (2004). State of the art biogranulation technology for wastewater treatment. Biotechnol Adv., 22(7):533-563.
18. Rajeshwari, K.V., Balakrishnan, M., Kansal, A., Kusum Lata, and Kishore, V.V.N. (2000). State-of-the-art of anaerobic digestion technology for industrial wastewater treatment. Renew Sustainable Energy Rev., 4(2): 135-156
19. O., Kizilgun F., Yilmazer G. (1996). Inhibition effects of zinc and copper on volatile fatty acid production during anaerobic digestion. Environ Technol., 17(11): 1269-1274
20. Manyi-Loh C.E., Mamphweli S.N., Meyer E.L., Okoh A.I. Makara G., Simon M. (2013). Microbial Anaerobic Digestion (Bio-Digesters) as an Approach to the Decontamination of Animal Wastes in Pollution Control and the Generation of Renewable Energy. Int. J. Environ. Res. Public Health., 10: 4390-4417
Edited by Yenfang Koh, a student of Suzanne Kern in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2015.
