Riftia pachyptila Symbiosis with Thioautotrophic Bacteria
Introduction
The functioning of an ecosystem depends upon the presence of organisms that can fix carbon dioxide to organic carbon. In environments without solar radiation, primary production depends on the processes of chemolithoautotrophs – chemosynthetic organisms which oxidize inorganic compounds to synthesize the NADPH and ATP needed to reduce carbon dioxide. The Riftia pachyptila, commonly known as the giant tube worm, has taken advantage of the ability of such chemolithoautotrophs, specifically thioautotrophic bacteria, and serves as a model organism for the study of host-symbiont co-evolution in deep-sea ocean vents 14.
Habitat
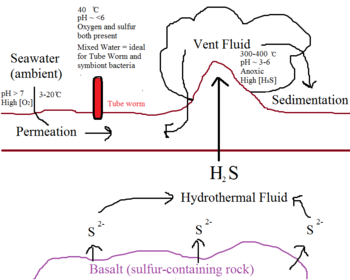
Hydrothermal vents occur in fissures on the seafloor, especially around active volcanoes14. Seawater seeps into these vents, circulates within the earth’s crust, and escapes back onto the surface as superheated vent fluid14. This vent fluid, which often reaches 300-400 ̊C in temperature, contains a high concentration of hydrogen sulfide from the reduction of sulfate by geothermal activity and interaction with sulfur-containing rocks such as basalt6. Vent sites are characterized by high acidity (pH 3-6), low oxygen levels, and extremely high temperatures14. In contrast, the ambient water surrounding the vent sites is cold and relatively rich in oxygen14. The Riftia pachyptila thrive at the interface between these two extremities, at a pH of around 6 and a temperature of 40 ̊C14. However, due to the complex interaction between populations of microbial organisms, the vent sites are rarely stable in flow rate, temperature, and sulfide concentrations9,11. To exploit this sporadic environment, both the host and the symbiont have developed interdependencies and have co-evolution.
Thioautotrophic mutualism
Thioautotrophic bacteria obtain energy needed for biosynthesis via sulfide-oxidation, which requires the presence of both sulfur and oxygen14. This poses an interesting challenge in the seafloor habitat, because sulfur and oxygen are distributed in distinctive zones14. High concentrations of dissolved sulfur are only present in extremely hot vent fluid, while oxygen is found in the cold, ambient seawater14. In addition, sulfur reacts spontaneously with oxygen to form oxides, making it even more inaccessible to thioautotrophs14. Although this oxidation process happens at a slower rate than biological fixation of sulfur, it nevertheless decreases its availability10,13. This is why most thioautotrophs are restricted to the interface between the ocean and the atmosphere to compete for binding to available oxygen14. Thioautotroph symbionts, however, have uniquely adapted to their environment byassociating with a protective environment, i.e. a host14. As a result, they are able to occupy a niche far away from the fierce competition happening at the ocean surface.
Acquisition of Thioautotrophs
As the tube worm matures from the juvenile stage, it seals the thioautotroph bacteria within itself by losing its mouth and developing a special organ called the trophosome4,7,12.
Sulfide Acquisition and Nutrient Exchange
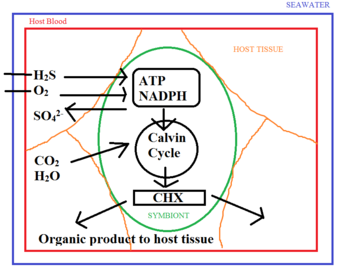
To provide the symbiotic bacteria with the nutrients they need, the tube worm synthesizes special haemoglobin that binds hydrogen sulfide independently of oxygen1,2,5,16. The worm’s circulatory system then delivers it to the bacteria residing in its trophosome14. In contrast to the haemoglobin present in humans and other vertebrates, this special haemoglobin is not inhibited in its ability to bind oxygen after binding to sulfur15. As a result, the worm is able to provide the bacteria with both the sulfur and the oxygen needed without allowing the two to spontaneously react with each other14. Within the trophosome, the thioautotrophs use hydrogen sulfide and oxygen to synthesize the NADPH and ATP needed for the reduction of carbon dioxide to organic carbon14.
The transfer of organic C from symbiont to host occurs through two possible mechanisms14. One possibility is that the worm subsists on organic secretions in the form of soluble organic molecules14. The other possibility is that the worm directly digests the bacteria14. Radio-labelling experiments done by Felbeck and Jarchow (1998) showed that both are likely occurring. When purified symbionts were incubated in the presence of labelled bicarbonate, it was found that labelled sugars and amino acids were excreted into the surroundings8. On the other hand, recent pulse-chase labelling analysis has shown a progressive appearance of labelled carbon in the host tissue occurring concurrently with a loss in the trophosome3. This suggests that digestion is another means by which organic carbon is being consumed in exchange for providing the symbionts with hydrogen sulfide and oxygen.
Evolutionary Perspective
The thioautotrophic bacteria that live in a mutually beneficial relationship with Riftia pachyptila belong to gammaproteobacteria14. The co-evolutionary dynamics between the host and this symbiont depend largely on what is called the symbiont transmission strategy14. In the case of sulfur-oxidizing bacteria, this strategy could be environmental, horizontal, or vertical14. Of these three strategies, environmental refers to the direct acquisition of symbionts from a free-pool of bacteria and has been proposed to be the most likely method by which this evolution occurred14. However, more research is needed before a conclusive result could be reached14.
Challenges and Future Directions
Many questions still remain unanswered in this model ecosystem of a deep-sea host organism and its chemosynthetic symbiont14. Particularly perplexing is the exact mechanism by which the symbiont is acquired by the tubeworm as well as processes of growth and metabolism14. Furthermore, the genetic dispersal of the bacterium as well as the details of the host-symbiont co-evolution are still under debate, especially when hindered by the inability to culture these bacteria apart from the host14. To solve these perplexing issues would require the examination of not only genomic sequencing, but also in situ studies that can accurately assess the gene-gene interactions between host and symbiont as well as their co-evolution14.
References
1Arp AJ, Childress JJ, Fisher CR. “Blood gas transport in Riftia pachyptila.” Bull Biol Soc Wash, 1985, 6:289–300
2Arp AJ, Childress JJ, Vetter RD. “The sulphide-binding protein in the blood of the vestimentiferan tube-worm, Riftia Pachyptila, is the extracellular haemoglobin.” J Exp Biol, 1987, 128:139–158
3Bright M, Keckeis H, Fisher CR. “An autoradiographic examination of carbon fixation, transfer and utilization in the Riftia pachyptila symbiosis.” Biology, 2000, 136:621–632
4Cavanaugh CM, Gardiner SL, Jones ML, Jannasch HW, Waterbury JB. “Pro karyotic cells in the hydrothermal vent tube worm Riftia pachyptila.” Science, 1981, 213:340–342
5Childress JJ, Fisher CR, Favuzzi JA, Kochevar RE, Sanders NK, Alayse AM. “Sulfide-driven autotrophic balance in the bacterial symbiont-containing hydrother mal vent tubeworm, Riftia pachyptila.” Biol Bull, 1991, 180:135–153
6Elderfield H, Schultz A. “Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean.” Annu Rev Earth Plant Sci, 1996, 24:191–224
7Felbeck H. “Chemoautotrophic potential of the hydrothermal vent tube worm, Riftia pachyptila.” Science, 1981, 213:336–338
8Felbeck H, Jarchow J. “Carbon release from purified chemoautotrophic bacterial symbionts of the hydrothermal vent tubeworm Riftia pachyptila.” Physiol Zool, 1998, 71:294–302
9Johnson KS, Childress JJ, Beehler CL. “Short-term temperature variability in the Rose Garden hydrothermal vent field – an unstable deep-sea environment.” Deep Sea Res, 1988a, 35:1711–172
10Johnson KS, Childress JJ, Hessler RR, Sakamoto-Arnold CM, Beehler CL. “Chemical and biological interactions in the Rose Garden hydrothermal vent field, Galapagos spreading center.” Deep Sea Res, 1988b, 35:1723–1744
11Johnson KS, Childress JJ, Beehler CL, Sakamoto CM. “Biogeochemistry of hydrothermal vent mussel communities – the deep sea analog to the intertidal zone.” Deep Sea Res, 1994, 41:993–1011
12Jones ML. “Riftia pachyptila Jones: observations on the vestimentiferan worm from the Galapagos Rift.” Science, 1981, 213:333–336
13Millero FJ, Plese T, Fernandez M. “The dissociation of hydrogen-sulfide in sea water.” Limnol Oceanogr, 1987, 33:269–274
14Stewart, FJ, Cavanaugh, CM. “Symbiosis of Thioautotrophic Bacteria with Riftia pachyptila.” Prog Mol Subcell Biol., 2006, 41:197-225.
15Weber RE, Vinogradov SN. “Nonvertebrate hemoglobins: functions and molecular adaptations.” Physiol Rev, 2001, 81:569–628
16Zal F, Lallier FH, Wall JS, Vinogradov SN, Toulmond A. “The multihemoglobin system of the hydrothermal vent tube worm Riftia pachyptila.1. Reex amination of the number and masses of its constituents.” J Biol Chem, 1996, 271: 8869–8874