The Role of Epstein-Barr Virus in Burkitt's Lymphoma
By Mary Frazier Greene
Introduction
Epstein Barr Virus (EBV) is among the most common human viruses and affects humans all around the world. EBV is a member of the herpes virus classification, where it is known as herpes virus 4. EBV is most known for causing mononucleosis, but it can cause other illnesses as well. EBV is most commonly spread through contact of bodily fluids of other infected humans. EBV specifically spreads most often through saliva, shared through kissing, sharing drinking vesicles, and toothbrushes. Additionally, EBV can be spread through contact with infected blood in medical procedures and semen during sexual contact. The virus is present in two stages: an active state and latent state, and the virus can only be spread during the active state. The virus can alternate between these stages to cause infection and then revert to latency. To diagnose an individual with EBV, blood tests are required to detect antibodies. Approximately 90% of adults have antibodies constitutively present in their blood, indicating a latent or active EBV infection. Symptoms of EBV infection include: fatigue, fever, sore throat, swollen lymph nodes (specifically in the neck), enlarged spleen, swollen liver, and a rash. However, individuals can be infected with EBV and not exhibit symptoms. After infection, the virus reverts to the latent stage. The virus can reactivate and cause symptoms again, but it is unlikely in individuals with healthy immune systems.(1)
Almost every adult in the world is a carrier for Epstein-Barr Virus, yet it typically only presents major health issues in those who have compromised immune systems. Individuals who are infected with EBV as children often display no symptoms, whereas adolescents, teenagers, and young adults who acquire the virus often fall ill with infectious mononucleosis. After acute illness, a small number of B-lymphocytes continue to carry the viral genome in a latent state. The immune system constantly works to suppress the infectious state of the virus by recognizing and ridding the body of viral cells that express proliferation driving genes.(2)
Alternatively, EBV is associated with malignant tumors, and in particular EBV is associated with Burkitt’s lymphoma. EBV association with Burkitt’s Lymphoma is especially prevalent in immunocompromised individuals. In fact, it was in a malignant tumor of B-cell origin that EBV was first discovered in 1964. Subsequently, studies have shown that EBV has the ability to immortalize B-lymphocytes by hindering apoptosis signals within the cell. The virally encoded cells, therefore, proliferate to high numbers in the body leading to malignancies. EBV can subsequently change the B-lymphocytes to lymphoblastoid cell lines. Individuals who carry the virus in most cases do not go on to have Burkitt’s Lymphoma because the immune system can recognize the transformed cells and destroy them before they cause malignancies. The immune system in immunocompromised patients, however, is not typically able to perform such a task, making the individual at risk for malignancies that lead to Burkitt’s lymphoma.(2) Furthermore, three forms of Burkitt’s Lymphoma are known to exist. One form is the endemic form that is typically found in children in the rain forest region of tropical Africa and Papua New Guinea. In these cases, 100% of the tumor cells contain the EBV infection. Burkitt’s Lymphoma in association with EBV is also present in sporadic form, typically in children, and includes differing degrees of EBV infection of the tumor cells. The last known form of Burkitt’s Lymphoma in association with EBV is found in patients who have AIDS in the adult population. AIDS-Burkitt’s Lymphoma is found to be common in equatorial areas of Brazil, where EBV infection rates and tumors are positively correlated.(5) The current research on this topic as well as specific data is described below.
Structure and Replication
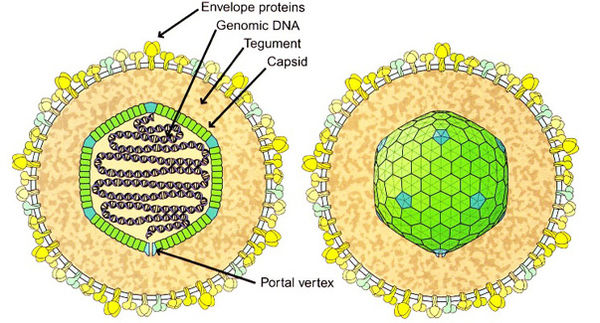
Herpes viruses, including Epstein Barr Virus, have a structure described as a capsid containing the double stranded DNA. The capsid is icosahedral in shape, meaning it has 20 triangular sides. An image of the virus is shown if Figure 1. Each triangle contains at least three identical but asymmetrical protein units; there is threefold symmetry around the axis through two opposed triangular faces; there is fivefold symmetry around an axis through opposite points; and there is twofold symmetry around the axis through opposite edges. The lipid envelope is made from host proteins, mainly intracellular membranes such as the nuclear membrane and the endoplasmic reticulum. The tegument (the space between the envelope and the capsid) contains both viral and host proteins, which include enzymes that interact with the host cell to hinder its defense responses during infection. The DNA is packaged in the icosahedral capsid, where it is spooled and tightly packed. The spike proteins around the edges attach the virus to the host cell to allow it to infect the cell. This structure appears to have evolved from a cell. The genome enters the host cell via fusion with the host cell membrane, and the capsid enters the cell. The DNA then enters the nucleus when the capsid injects the DNA into the nuclear pore.(3)
The replication cycle of Epstein Barr Virus and other Herpes viruses is done by DNA integration with the host cell DNA and the lytic cycle. The virion attaches to the outer membrane of the host cell, which is enabled by the “spikes” on the outer membrane of the virus structure. There are two phases that the viral genome can undergo once it enters the host cell’s cytoplasm: Lytic or latent life cycles. In the lytic cycle, the phage plasmid replicates quickly using the host cells proteins to replicate (such as polymerases and ribosomes). Capsids are also formed to incase the newly replicated genomes. Once the “late” gene is expressed in the replication process, the cell lyses and the newly formed phages are released and able to infect more cells exponentially.(3) Alternatively, the viral genome can undergo the latent cycle. This occurs when the viral genome uses the machinery of the host cell to perform continual replication of the viral genome. The replication of the EBV in the cell relies entirely on the host cell’s replication tools. Once stress incurs, the viral genome can cause cell lysis via the lytic cycle, which is always lethal to the cell.(3) Epstein Barr Virus has a unique way of taking advantage of the host cells during the latent stage. The virus is often found in B cells, where the viral plasmid uses the host cells replication tools to continually replicate the plasmid. Furthermore, the infection of the B cells by EBV triggers the expression of certain genes that result in uncontrolled cell proliferation of the infected B cells, ensuring immortality of the virus in the infected cells by disruption of the cells ability to undergo apoptosis.(4)
Current Research

EBV Interactions with Human B-lymphocytes2:
Figure 2 illustrates the role of certain Epstein-Barr Virus encoded proteins in the latent infection of the host cell, in particular the infection of B lymphocytes. Latent infection of B lymphocytes by EBV has been connected to occurrences of cancer, specifically malignancies in lymphoma. One specific connection found between EBV and lymphoma has been discovered in cases of Burkitt’s Lymphoma that is endemic to the rain forest regions of tropical Africa. EBNA is a nuclear antigen that has been discovered to be expressed in all cells that have been infected with EBV, and it was subsequently termed EBV-encoded nuclear antigen (EBNA).(2)
EBNA-1 is a DNA binding protein that plays a crucial role in ensuring continual replication of the viral genome in the host cell. EBNA-1 is important in the division of the EBV genome during the cell division stage of the replication cycle of the host cell.(2)
EBNA-2 is crucial for cell proliferation. EBV is known to increase cell proliferation by hindering the host cell’s ability to regulate and undergo apoptosis. EBNA-2 doesn’t interact with DNA directly, but instead it interacts with both viral and cellular promoters to enable continual cell proliferation. The expression of EBNA-2 requires specific transcription factors in B-cells, including PAX5 and OCT2. EBNA-2 also works closely with RBP-Jκ, which binds to a specific DNA sequence that is involved in the Notch signaling pathway. Notch genes are responsible for regulating the development of certain cell types, and mutations in the Notch signaling pathway is known to lead to the development of malignant cells in humans.(2)
EBNA-3, 4,6 (also known as EBNA-3A, -3B, and 3C, or the EBNA-3 family proteins) have similar genomic organization and are found in tandem on the genome sequence. Similar to EBNA-2, the EBNA-3 family proteins all associate with RBP-Jκ, and the combination of these proteins is involved in the regulation of transcription of the B-lymphocytes. EBNA-3 family proteins are also involved in the check-point parts of the cell replication cycle, which suggests the ability to influence the transcriptional regulation of the cell. The EBNA-3 family proteins are also involved in the expression of the Ah receptor in the cytoplasm of the cell. The Ah receptor is a receptor present in most, if not all, vertebrates and is involved in metabolizing toxic compounds in the body. The EBNA-3 family proteins influence the transcription of AhR responsive genes at both the basal level and in the presence of ligand binding to the Ah receptor. The EBNA-3 family proteins also bind to the XAP-2 regulatory subunits of the AhR complex, which is involved in the DNA tumor virus hepatitis B. When EBNA-3 is knocked out of the viral genome, B cells showed significantly slower growth than B cells infected with the wild type strain of the virus. EBNA-3 family proteins work closely with the 4-hydroxy-tamokifen (HT) dependent promoter, so that in the absence of the promoter, the virus is unable to immortalize the cell and increase proliferation. These findings strongly suggest that the EBNA-3 family proteins in particular play a crucial role in immortalizing the cell and increasing proliferation of the infected cells.(2)
The combination of these findings about each specific protein encoded by Epstein-Barr virus has shed light on the association of EBV with Burkitt’s Lymphoma. All Burkitt’s Lymphoma tumor cells have EBV present, whereas other cell lines in the body have lost the EBV infection. This unanimous presence in tumor cells is strong evidence for the understanding that EBV is essential for cell proliferation in the body. These cells in vivo only express EBNA-1 instead of the entire virus program, which suggests that EBNA-1 is sufficient to induce Burkitt’s lymphoma by counteracting the cells natural ability to undergo apoptosis. (2)
EBV Association with Pediatric Burkitt’s Lymphoma in a Single Institution in Argentina:(5)
Three forms of latency infection by EBV are known (I, II, and III). The difference between the types of latency correspond to the number and types of proteins expressed by the viral genome. Latency I is typically associated with Burkitt’s Lymphoma and it is shown to express EBNA 1, the Qp promoter, and the EBERs.(5) EBNA-1 and the EBERs work in combination to prevent apoptosis and ensure survival of the tumor cells. EBER stands for Epstein-Barr Virus encoded RNA, and EBERs are noncoding RNAs that are secreted by EBV infected cells, which ignite the immune response in the body and subsequent inflammation of the area, accounting for the pathogenesis of EBV. EBERs are found in all forms of latent infection of EBV.(6)

Figure 3 shows the results of EBERs in situ hybridization of tumor cells taken from patients retrospectively at an institution in Buenos Aires, Argentina from 1990 to 2008. The researchers used monoclonal antibody anti-FITC labeled with alkaline phosphatase in this in situ hybridization study to identify the presence of EBERs in the cells. The positive control was an in situ hybridization performed on Hodgkin’s lymphoma Hodgkin Reed Sternberg cells. Additionally, immunohistochemical staining was performed on FFPE tissues to analyze the presence of EBV and Burkitt’s lymphoma. The antibodies used in this staining were: CD3, CD10, CD20, CD30, CD45, Bc16, ALK, Ki67, Bc12, c-Myc, and LMP1. Panel A and B show two examples of in situ hybridization of tumor tissues with the presence of EBERs, thus confirming the presence of Epstein-Barr Virus infection in the tumor cells. Panel C is the image of the in situ hybridization of the control tissue taken from Hodgkin’s lymphoma Hodgkin Reed Sternberg cells. Panel D shows the immunhistochemistry analysis of the LMP1 antigen expression. The study showed that LMP1 expression is localized at the cytoplasmic membrane, as is evident in the image above. Immunohistochemistry could not be performed for other EBV encoded proteins due to the lack of available antibodies associated with these antigens.(5)

Figure 4 shows RT-PCR analysis of the RNA encoding EBERs expression studied via PCR amplification. This analysis aimed to study the expression of EBERs, EBNA-1 promoter usage, LMP2A, BZLF1, and BHRF1 RNA. Part A of Figure 4 shows the good quality RNA bands confirmed by PGK expression in 9 out of the 10 samples analyzed.
The distribution of EBV presence in Burkitt’s lymphoma tumor cells in this study was found to be sporadic, where 29% of the immunocompetent patients were found with an EBV-Burkitt’s Lymphoma association. This finding was lower than was expected, and the researchers attributed this deviation from expectation to the fact that all age groups were included in other studies that produced higher percentages of association, and the fact that both immunocompetent and immunosuppressed patients were often not distinguished in these other studies. The association between EBV and Burkitt’s lymphoma was found to be higher in the northern part of Argentina, as well as higher in pediatric groups as opposed to adult groups. It has been proposed that the higher association rate in the pediatric group could be a result of a complication in EBV infection in young children (less than 10 years), which subsequently led to the development of Burkitt’s lymphoma. Part B of Figure 3 shows the distribution of this association in a spectrum of age groups. As illustrated in the figure, children under the age of 4 are found to have a high prevalence of this association, whereas little to no association is found in all older age groups. Lara et. al. (2014) explains that a basal level amount of EBV latent antigens enter the germinal center of the B cells in HIV patients, where the B cells express the latent EBV infection constitutively to ensure continued cell proliferation and, therefore, continual replication of the viral genome. Furthermore, Burkitt’s lymphoma cells are found to be derived from the germinal center of the B cells, and are thus often found in association with EBV infection. Thus, the inability of B cells in HIV infected patients to shut off the expression of the viral genome gives rise to LMP1 expression from the germinal center of the cell. HIV infected patients are immunosuppresed where the cells are unable to undergo check points in replication, so unhindered proliferation of EBV-infected lymphocytes can take place.(5)
Future Studies
Epstein-Barr Virus is known to cause illnesses including infectious mononucleosis, Burkitt’s lymphoma, Hodgkin’s Lymphoma, nasopharyngeal carcinoma, gastric cancer, lymphoma common in transplant patients, and numerous other types of cancer. This chilling association with life threatening illness has lead researchers to explore the possibility of developing preventative measures, especially because over 90% of the adult population worldwide is infected with the virus. Vaccines have proven successful in the prevention of other viral illnesses, including influenza and human papillomavirus (HPV). The development of a vaccination against EBV would not only spare countless people from enduring infectious mononucleosis each year, but would also reduce the rate of diagnosis of specific forms of cancer by hundreds of thousands each year. Specifically, the development of such a vaccine could prevent up to 200,000 new cases of cancer every year. Epstein-Barr Virus was discovered 50 years ago (in 1964) by Sir Anthony Epstein, and studies that followed its discovery showed that it was the first known human tumor virus. There are difficulties with creating a vaccine for EBV because it is a different type of virus than the human papillomavirus, and it is transmitted differently.(7) Furthermore, a development of an EBV vaccine has been in the works for a long time because it is challenging to establish a suitable animal model, and researchers working on the project do not agree on the exact goals of the vaccine. Some argue that the vaccine should first and foremost aim to prevent Burkitt’s lymphoma, whereas others want to relieve the ailment of the population that acquires infectious mononucleosis. Attempts to zero in on a suitable vaccine have thus far come up short in the final stages. Additionally, it has been reported that EBV infection that has manifested as infectious mononucleosis or other illnesses can permanently damage the immune system by causing a deficit in T cell responsiveness to interleukin. Therefore, some researchers want to aim to limit this damage done to the immune system by EBV with the developed vaccine. This type of vaccine would aim to prevent the manifestation of EBV infection as infectious mononucleosis, but it would not help in the acquirement of asymptomatic EBV. It is argued that this damage to the immune system as a result of infectious mononucleosis is at the foundation of the development of malignant tumors associated with EBV infection. This proposed correlation between the damage done to the immune system by infectious mononucleosis has not yet been sufficiently proven, however, and so some researchers argue that they do not want to risk developing a vaccine that ultimately does not serve the purpose of preventing the development of malignant tumors because an incorrect mechanism was identified and pursued. Thus far, a gp350 vaccine has been developed, but it is still undergoing trial. It has been shown to reduce the severity of infectious mononucleosis, but it has also been associated with immune complex reactions including arthritis of the ankles, knees, and back. This could possibly be a consequence of individuals receiving the vaccine who already have EBV antibodies, so further studies are being performed on animal models to see the effects of the vaccine on previously uninfected individuals.(8)
References
1. Epstein-Barr Virus and Infectious Mononucleosis. Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases. January 2014. http://www.cdc.gov/epstein-barr/about-ebv.html.
2. Klein, George, Eva Klein, and Elena Kashuba. "Interaction of Epstein-Barr Virus (EBV) with Human B-lymphocytes." Biochemical and Biophysical Research Communicaitons 396 (2010): 67-73
3. Slonczewski, Joan L., and John W. Foster. Microbiology: An Evolving Science. 3rd ed. N.p.: W. W. Norton, 2014.
4. Yates, John L. "Epstein-Barr Virus DNA Replication." DNA Replication in Eukaryotic Cells. N.p.: Cold Spring Harbor Laboratory, 1996. 751-68.
5. Lara, Julia, Melina Cohen, Elena De Matteo, Luis Aversa, Maria Victoria Preciado, and Paola Chabay. "Epstein-Barr Virus (EBV) Association and Latency Profile in Pediatric Burkitt's Lymphoma: Experience of a Single Institution in Argentina." Journal of Medical Virology 86 (2014): 845-50.
6. Iwakiri, Dai, and Kenzo Takada. "Role of EBERs in the Pathogenesis of EBV Infection." Advances in Cancer Research 107 (2010): 119-36.
7. "Developing a Vaccine for the Epstein-Barr Virus Could Prevent up to 200,000 Cancers Globally Say Experts." Cancer Research UK. Cancer Research UK, 24 Mar. 2014.
8. Balfour, Henry H. "Epstein-Barr Virus Vaccine for the Prevention of Infectious Mononucleosis-- And What Else?" The Journal of Infectious Diseases 196 (2007): 1724-726.
9. Giovanonni, Gavin. "Multiple Sclerosis Research: Research: EBV Persistence in B Cells." Multiple Sclerosis Research: Research: EBV Persistence in B Cells. Barts and the London School of Medicine and Dentistry, 15 Sept. 2012. Web. 05 May 2014.
