Toxoplasma gondii and Schizophrenia
By Sara Myers
Introduction
While the field of biological psychiatry aims to investigate many mysteries of why humans act the way we do using biology-based studies, many questions remain unanswered. The protozoan Toxoplasma gondii is an obligate intracellular parasite whose habitat consists of cells of endotherms. T.gondii belongs to the phylum Apicomplexa, and all species in this phylum are parasitic and possess an apicoplast, which is a unique plastid organelle containing a structure that allows penetration of host cells. All Apicomplexa exclusively parasitize animals, and they are all unicellular and have the ability to form spores.
Although T.gondii can live and asexually reproduce in all endotherms, they can only sexually reproduce in the digestive tracts of felids, a family including all cats, thus the definitive hosts of T.gondii are cats while other animals are intermediate hosts and carriers. T.gondii is often stored in cysts in warm-blooded animals’ tissues, allowing the parasite to be transmitted via consumption of cyst-laden tissue. The infectious cycle may begin when a cat consumes a mouse whose tissues contain cysts of T.gondii, as the parasite can then sexually produce oocysts (cysts composed of zygotes) in cats’ intestinal epithelial cells (Fig. 1).
When these cells burst, the oocysts are released into the intestines and excreted in the feces, which may then contaminate food, soil, water that may be consumed by other animals. Humans who subsequently consume meat of animals whose tissue holds cysts of T.gondii may become infected; handling litter boxes of infected domestic cats also serves as a trajectory for human transmission (Fig. 1). T.gondii have developed sneaky evolutionary tactics for infecting intermediate hosts, as the moment an oocyst is ingested by a human, proteolytic enzymes degrade the cell wall and release the parasite to enter host cells.
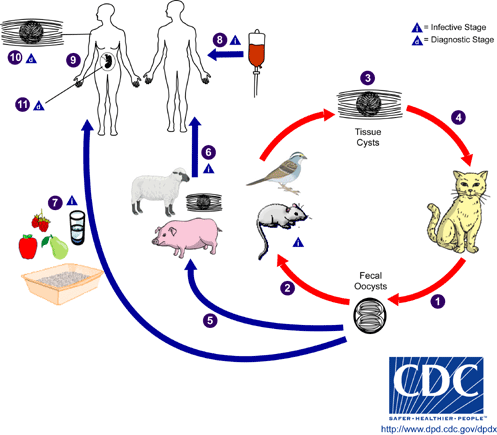
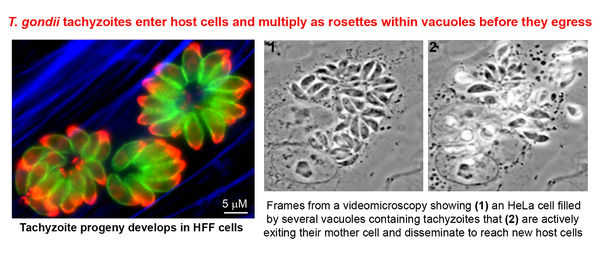
At this stage, the parasites develop into motile tachyzoites, which can quickly reproduce asexually and sequester themselves in parasitophorous vacuoles, which they create upon entry to the cell. Once the cell dies and bursts, the replicated parasite travels to all tissues of the body by way of circulating blood. Thus, the parasite reaches the brain and spinal fluid, where cysts form and store T.gondii in humans. Humans may remain chronically infected for the duration of their lives, as cysts periodically rupture and re-form; a resulting third of the world’s population is chronically infected with T.gondii.
Upon initial infection, healthy humans generally do not develop any symptoms, but immunocompromised individuals are likely to develop toxoplasmosis, a disease that may cause permanent damage to the central nervous system as well as tissue of essential organs. Toxoplasmosis may also cause neurological diseases and in particularly severe cases, death. People at highest risk for developing acute toxoplasmosis include babies and young children, pregnant women, people infected with HIV/AIDS, recent organ recipients and people being treated with chemotherapy. The neurological disease most commonly associated with toxoplasmosis and latent T.gondii infection is schizophrenia, and multiple case-control and population based studies have presented data on this relationship. Although many studies have presented correlations between T.gondii and schizophrenia, evidence is limited and many questions regarding causality remain.
Methods For Diagnosing Infection
The agglutination screen has been deemed appropriate when testing for the presence of antibodies produced by the immune system in response to certain infections. Additionally, the Sabin-Feldman dye test is widely used to test for toxoplasmosis, as it uses methylene blue dye, which is unable to penetrate antibodies to T.gondii. Thus, antibodies to T.gondii will remain unstained while other antibodies appear blue as a result of the stain. IgM antibodies, which are produced sooner after infection and disappear sooner than IgG antibodies, are often tested for using enzyme-linked immunosorbent assays. These assays use antibodies bound to enzymes in order to bind relevant antigens and substrates, inducing a color change in the enzyme’s substrate. If no IgM antibodies exist in a given sample, substrate and antigens will remain unbound and no color change will occur.
Pregnancy and transfer to fetus
If a pregnant woman becomes infected with toxoplasmosis, there is a chance the fetus will develop congenital toxoplasmosis as a result of transfer of the parasite itself through the placenta. Congenital toxoplasmosis may result in eye damage, hearing loss, and vision problems as well as a breadth of neurological and central nervous system disorders. [6] But, even when the fetus does not develop congenital toxoplasmosis and is born apparently healthy, multiple studies have shown that offspring born to infected mothers may be at risk of developing schizophrenia as a child as well as later in life.
When a woman becomes pregnant and her blood serum is drawn, it may be examined for antibodies to T.gondii, as this serves as the most direct approach to diagnosis of toxoplasmosis as well as an effective means of studying resulting effects of an infection. A positive titer suggests the woman has been exposed to T.gondii before and is likely immune to toxoplasmosis. Alternatively, if a woman is exposed to T.gondii during pregnancy, the fetus is at risk for toxoplasmosis by route of placental transmission. Although in these cases the fetus may be morphologically damaged, particularly within the central nervous system, children born to serologically positive mothers with high concentrations of T.gondii antibodies are more likely to develop schizophrenia as adults than children born to mothers with low or no T.gondii antibody concentrations.
The initial findings to suggest that maternal exposure to toxoplasmosis could lead to development of schizophrenia in adult offspring came from a case-control study in 2005, in which researchers conducted serological assays from maternal serum pertaining to offspring who developed schizophrenia later in life [1]. This study was based on the knowledge that fetal brain damage may occur if while pregnant, the mother becomes infected with toxoplasmosis. [3] By obtaining archived serum taken from pregnant women and matching the serum with the corresponding adults with schizophrenia, the researchers were able to conduct assays testing for elevated IgG and IgM antibodies to T.gondii. To determine IgG concentration, Brown et al. performed a screen agglutination test, with a follow-up Sabin-Feldman dye test for confirmation. In sera containing positive IgG titers, IgM antibody levels were tested for in order to determine whether the toxoplasmosis infection was recent. This was done using a double-sandwich enzyme-linked immunosorbent assay.
This paper also developed a categorization system for quantifying positive IgG titers, in order to insure sufficient numbers of subjects in each test group as well as to allow for investigation of association with schizophrenia at different magnitudes. Their results indicated that serum from older mothers tended to have higher IgG concentrations, but after adjusting for age, they found that serum with high IgG titers correlated positively with offspring with schizophrenia. Additionally, the odds ratio for the development of a schizophrenia spectrum disorder for these subjects was 2.61, in comparison the odds of 1.00 for the neutral baseline reference group. Since no subjects had positive IgM titers, correlation is not a result of active primary infection. The possibility of high IgG levels due to a reactivated infection may not be dismissed, as the prevalence of reactivated dormant microcysts is currently unknown. This study presents results suggest that prenatal exposure to toxoplasmosis is indeed a risk factor for the development of schizophrenia. [1]
Another case-control study testing whether T.gondii may be a risk factor for schizophrenia was done in Denmark, although this study focused specifically on early-onset schizophrenia. [2] Early-onset schizophrenia is categorized as a schizophrenia spectrum disorder in people below the age of 18. [4] The researchers conducted similar assays as in the experiment down by Brown et al., but used serum taken from heel-pricks of newborns. [1, 2] They found that increased levels of T.gondii IgG antibodies correlated significantly with the development of childhood schizophrenia. This study replicated the results presented by Brown et al., but used a sample size three times larger, used blood samples of newborns, and was able to compare IgG levels in mothers’ blood with IgM levels in babies. This serves as an important comparison when assessing the relationship between maternal biological risk factors and subsequent infections in offspring. This study also only investigated a narrowly defined variety of schizophrenia, while other studies have included any disorder on the schizophrenia spectrum or even any form of psychosis. Another extremely important aspect of this study was its access to the PKU biobank, which registers all people born in Denmark and links neonatal samples to multiple other nationwide databases. Thus, they were able to investigate whether results were due to various confounding factors including family history of schizophrenia, urbanicity of birth location, year of birth, and gender. [2]
Multiple other studies have replicated this data and found positive associations between maternal toxoplasmosis and subsequent schizophrenia in offspring. Unfortunately, it is extremely difficult to establish causality because of the latent period between original neonatal infection and development of schizophrenia later in life. The incidence of schizophrenia may potentially be reduced if pregnant women are able to implement prevention methods for Toxoplasmosis.
Mechanism of contribution to risk of schizophrenia
Evolutionarily, T.gondii is very successful, as it has managed to infect a third of the world’s population and has developed advanced mechanisms for avoidance of host immune response. Furthermore, as T.gondii can only sexually reproduce in cats, the parasite has evolved to manipulate the behavior of mice in a way that decreases the mice’s sense of predation risk, increasing the likelihood of predation by cats. Once the cat consumes the infected mouse, the parasite may sexually reproduce in its intestines, completing the infectious cycle. In contrast to many other behavior-altering parasites, T.gondii does not appear to alter any other behavioral or physical aspects of the mouse host. Not only has T.gondii been shown to cause mice to increase activity in conjunction with a decrease in neophpbia related activities, but evidence now exists to suggest T.gondii is able to alter rats’ innate avoidance of cat odor.

Mice who have been isolated from cat odor for over a hundred generations demonstrate extreme anxiety and aversion to the scent, but mice infected with T.gondii have these aversion mechanisms dismantled, and are often significantly attracted to cat odor. [7] This has lead researchers in the field to label infected mice as “suicidal”, which correlates strongly with behavioral changes in humans, including increased rates of suicide in women with latent or acute toxoplasmosis.
An experiment completed in 2000 investigated whether infected rats lacked aversion to cat odor by counting the number of times rats visited corners of outdoor pens containing cat urine (Fig. 3). Rabbit urine, the rat’s own urine and water were used in the other three corners, and the rats visited the corner containing cat scent significantly more than they visited the rabbit corner and the neutral corner. Additionally, uninfected and infected mice differed only in the amount they visited the cat corner. This remained the only behavioral pattern affected by the parasite, while all other behavioral categories remained unchanged and the overall health of the infected mice was comparable to that of the uninfected mice. Since up to 84% of people infected with T.gondii in the United Kingdom, where this study was done, exhibit altered brain function, these results may elucidate information regarding the mechanism by which T.gondii alters human neurological activity. Also, since rats and humans both exhibit the omnivore’s paradox, in which a balance between exploration and avoidance of risk must be struck, the mechanism of neurological alteration is likely similar. Since the parasite has evolved to inhabit and manipulate a mammal, it has been speculated that the parasite is not selective, hence its contribution to neurological disease, increased suicide rates, and schizophrenia in humans. [7]

Another very important recent experiment demonstrated that anti-psychotic drugs and mood-stabilizers inhibit the asexual reproduction of tachyzoites (fully developed, quick-dividing T.gondii) in culture. In this experiment, the researchers tested and compared the effects of haloperidol, valproic acid, Dapsone and water on inhibition of tachyzoite replication prior to cyst development. This procedure was chosen due to the fact that once the parasite sequesters itself in cysts in the brain and extracellular space (in the spinal cord, heart and muscles) the tachyzoites develop into bradyzoites, which are durable, reproduce slowly, and stay alive longer.
Based on these in vitro results, Webster et al. (2006) tested the effect of anti-psychotic and mood stabilizing drugs on mice infected with T.gondii. [8] This study tested whether infected mice treated with haloperidol, an anti-psychotic drug commonly used to treat schizophrenia, and valproic acid, a mood stabilizer often used in conjunction with other drugs to treat schizophrenia, exhibited reduced “suicidal” attraction to cats and maintained perception of feline predation risk. They found that these drugs were as effective and in some cases more effective than the widely accepted toxoplasmosis antibiotic, Dapsone. The results of this experiment indicated that not only did infected mice treated with haloperidol, valproic acid and Dapsone visit the corner with cat odor fewer times, but they also spent less time there when they did visit. While all three drugs reduced infected mice’s cognitive and behavioral alterations, haloperidol was most effective, while Dapsone was less effective and valproic acid was the least effective. Haloperidol and valproic acid serve as calcium channel blockers, and since tachyzoites depend on calcium transport via ion channels to invade host cells, invasion of brain cells by the parasite was reduced. Additionally, both toxoplasmosis and schizophrenia are associated with heightened dopamine levels, and haloperidol has dopamine antagonist properties. These results are highly applicable in the search for a causal link between T.gondii and schizophrenia, as well as a way to prevent T.gondii infection from affecting cognitive and behavioral function or development of schizophrenia (Fig. 4).

Genetics
The life cycles of various pathogens depend on selective genetic expression, and viral pathogens have been found to alter expression of genes they depend on for production of essential proteins used for metabolism. Many of the genes associated with schizophrenia, including those that closely regulate levels of nutrients in the blood as well as neurophysiological genes, also affect the life cycle of T.gondii. Numerous schizophrenia susceptibility genes are partially responsible for membrane components that T.gondii binds to directly prior to entering the host cell. The gene GNPAT codes for expression of proteins that metabolize glycerone-3-phosphate to acylclyceronephosphate, which parasites may metabolize from the blood stream. Also, tryptophan is metabolized by many parasites including T.gondii and the gene that codes for tryptophan hydroxylase (TPH) is also related to expression of serotonin receptors. The gene coding for expression of NMDA receptors is very sensitive to concentrations of glutathione, which is depleted in cerebrospinal fluid and the brain in people with schizophrenia. This presents an advantage for T.gondii, since fewer NMDA receptors to bind glutathione allows for more available glutathione, which activates an apyrase released by T.gondii, causing the parasite to leave the vacuole it is contained in. This results in T.gondii quickly using up the host cell’s ATP and draining it of energy. [9] Various other genes metabolize and provide ligands that T.gondii binds, including PLA2, GALNT7 and B3GAT1.
MUTED is a gene that regulates transport of intracellular items to the lysosome and it is disrupted in people with schizophrenia, which may partially explain how T.gondii are able to stay in a parasitic vacuole and avoid transport to the lysosome. [9] In addition to the genes mentioned here, the presence of cysts of T.gondii in the brain and spinal fluid induces immune response genes to increase production of proinflammatory cytokines, with diffusion to blood plasma and cerebrospinal fluid. Elevated levels of these cytokines in the blood plasma (TNF) and cerebrospinal fluid (IL-6) have been closely associated with suicidal behavior. But, T.gondii infection is more closely related to attempting suicide and failing than actually committing suicide, which has led some experts in the field to connect failed but attempted suicide to schizophrenia. [10]
Conclusion
The seroprevalence of Toxoplasma gondii infection demonstrates the extreme evolutional success of the parasite. Very few parasites infect as much as a third of the world’s population, as this requires the ability to reproduce quickly, spread easily, and reside in a diverse variety of habitats. T.gondii can live in any warm blooded animal, as well as in contaminated water and soil. The transfer of the parasite to humans can thus occur in a number of ways, including drinking contaminated water, eating unwashed fruits and vegetables, eating undercooked meat with T.gondii cysts, and handling of domestic cat feces. But, cats are more likely to acquire T.gondii infections, which do not cause harm to the cats, as kittens. Therefore, immunocompromised people are sometimes made aware of this potential source of infection, although awareness of sources of T.gondii infection does not seem to be as prevalent as one might hope. Methods of transfer of the parasite between humans include blood and organ transfusions and from mother to fetus via the placenta.
T.gondii is able to live in parasitic vacuoles that are created upon entry into a host cell, and as these vacuoles have mechanisms of avoiding lysosomes, the parasites continue moving into new host cells when an old one dies. Additionally, although the parasite can reproduce asexually and change conformation according to necessary growth rate and nutrient uptake for a specific environment, the mechanism by which T.gondii alter the behavior of mice in order to be transferred into a cat is of particular importance. The only change T.gondii cause in mice appears to be a reduced or absent sense of predation risk, which all mice inherently have.
T.gondii represents one of the only parasites with such precise specificity of cognitive alteration, as most parasites alter multiple behavioral, cognitive, and physical aspects of their hosts. The mouse model also serves as a basis for research on neurological as well as physiological effects of the T.gondii infection in humans, a field of research that has flourished in the past fifteen years. Close associations have been found between toxoplasmosis, T.gondii infection and schizophrenia, and results show that even one’s latent infection that never produces symptoms could contribute to the chance of an adult offspring developing schizophrenia and other neurological and cognitive disorders.
As research indicating correlations between the parasite and neurological disorders advances, research searching for exact causative mechanisms will hopefully develop as well. Knowledge of the mechanism by which T.gondii contributes to schizophrenia and published causative data could potentially allow for the development of drugs that target this association, which may also raise international awareness of the dangers of T.gondii infection. Although the acquisition of untreated toxoplasmosis whilst pregnant often causes extremely unfortunate and permanent damage to the fetus, subtler effects like schizophrenia are highly common. Hopefully as research in this field moves forward, people will become more aware of available methods of preventing T.gondii infection, more countries will incorporate toxoplasmosis screening into procedural pregnancy screens, and accessible, publicized information will be made available about protecting oneself and one’s family.
References
1. Brown, A., Schaefer, C., Quesenberry, C., Liu, L., Babulas, V., Susser, E. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. 2005. American Journal of Psychiatry. Vol. 162(4): 767-773.
2. Mortensen, P., Norgaard-Pedersen, B., Waltoft, B., Sorenson, T., Hougaard, D., Torrey, E., Yolken, R. Toxoplasma gondii as a risk factor for early-onset schizophrenia: Analysis of filter paper blood samples obtained at birth. 2007. Biological Psychiatry. Vol. 61 (5): 688-693.
3. Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH: Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry 2001; 58:1032–1037.
4. Nordqvist, Christian. "What Is Childhood Schizophrenia? What Causes Childhood Schizophrenia?". 2010. Medical News Today.
5. Flegr, Jaroslav. Influence of latent Toxoplasma infection on human personality, physiology and morphology: pros and cons of the Toxoplasma-human model in studying the manipulation hypothesis. Journal of Experimental Biology. Vol 216: 127-133.
6. Pinon, J., Dumon, H., Chemla, C., Franck, J., Petersen, E., Lebech, M., Zufferey, J., Bessieres, M., Marty, P., Holliman, R., Johnson, J., Luyasu, V., Lecolier, B., Guy, E., Joynson, D., Decoster, A., Enders, G., Pelloux, H., Candolfi, E. Strategy for diagnosis of congenital toxoplasmosis: Evaluation of methods comparing mothers and newborns and standard methods for postnatal detection of immunoglobulin G, M, and A Antibodies. 2001. Journal of Clinical Microbiology. Vol. 39(6): 2267-2271
7. Berdoy, M., Webster, J. P., Macdonald, D. W. Fatal attraction in rats infected with Toxoplasma gondii. 2000. Proc. R. Society. Lond. Vol. 267(1452): 1591-1594.
8. Webster, J., Lamberton, P., Donnelly, C., Torrey, E. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite mediation on Toxoplasma gondii’s ability to alter host behavior. Proc. R. Society. Lond. Vol. 237(1589): 1023-1030.
9. Carter, C. Schizophrenia susceptibility genes directly implicated in the life cycles of pathogens: cytomegalovirus, influenza, herpes simplex, rubella and Toxoplasma gondii. 2009. Schizophrenia Bulletin. Vol. 35(6): 1163-1182.
10. Ling, V., Lester, D., Mortensen, P., Langenberg, P., Postolache, T. Toxoplasma gondii seropositivity and suicide rates in women. 2011. J. Nerv. Men. Dis. Vol. 199(7): 440-444.
