Salmonella enterica: foodborne illness: Difference between revisions
No edit summary |
|||
| (190 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | {{Curated}} | ||
[[Image:Industrial-Chicken-Coop.JPG|thumb|400px|right|[Figure 1]Commercial Poultry Farm.[http://www.google.com/imgres?imgurl=http://upload.wikimedia.org/wikipedia/commons/7/7c/Industrial-Chicken-Coop.JPG&imgrefurl=http://www.rootsimple.com/2007_08_01_archive.html&usg=__r-5Jp0OqjCQlw0G68JcqIdoVbdg=&h=1728&w=2304&sz=1556&hl=en&start=0&zoom=1&tbnid=WznmvC5_1IFGeM:&tbnh=132&tbnw=165&ei=AFmzTf7rM8_SgQfRkKTHCw&prev=/search%3Fq%3Dchicken%2Bfarms%26um%3D1%26hl%3Den%26client%3Dsafari%26sa%3DN%26rls%3Den%26biw%3D1264%26bih%3D616%26tbm%3Disch&um=1&itbs=1&iact=hc&vpx=974&vpy=135&dur=412&hovh=165&hovw=221&tx=107&ty=93&page=1&ndsp=18&ved=1t:429,r:5,s:0].]] The bacteria <i>Salmonella</i> is commonly associated with food poisoning in countries all over the world. There are two species of <i>Salmonella</i>: <i>S. enterica</i> and <i>S. bongori</i>. However, the species that most people refer to when they talk about <i>Salmonella</i> is <i>S. enterica</i>. This species is divided into a subset comprising of serovars. In <i>S. enterica</i> alone, there are over 2,000 serovars of bacteria. <i>Salmonella</i> infections can originate from household pets containing the bacteria, particularly reptiles, improperly prepared meats and seafood, or the surfaces of raw eggs, fruits, or vegetables that have not been adequately disinfected. The most common cause of food poisoning is associated with the serovar <i>S. Enteritidis</i>. These outbreaks are attributed to contaminated poultry, chicken eggs, and products that contain eggs. Because of the high demand for poultry and poultry products, farms can become highly unsanitary [Figure 1]. For example, chicken farms contain thousands up on thousands of chickens all living in the same area. Dead chickens are left for days before they are removed from the others. If one chicken contains the <i>Salmonella</i> bacteria, the organism can spread rapidly through thousands of others. When these chickens are processed and sent to stores all over the country, a mass <i>Salmonella</i> outbreak can occur [5]. When humans contract this disease, they can expel some of the <i>Salmonella</i> bacteria in their feces. In areas with poor sanitation, these bacteria can get into the water system where they can infect another person or animal and begin a whole new cycle of transmission. | |||
[[Image: | |||
<br>Poultry eggs can contain <i>Salmonella</i> bacteria both on the surface as well as inside. After hens lay eggs, they sit on top of them to keep them warm. While sitting, fecal matter can transfer from the chicken to the surface of the egg. If the chicken is infected with <i>Salmonella</i>, the eggs must be properly disinfected in order to prevent disease. This is a larger problem in developing countries rather than the US or UK. The more common method of transmittance in the US is attributed to eggs infected with <i>Salmonella</i> on the inside. <i>Salmonella Enteritidis</i> can infect the ovaries of a hen and thus contaminate the egg before the shell forms [6]. Because the bacterium silently infects the hen’s ovaries, the hen does not show any signs of an illness. Therefore, seemingly healthy hens transmit this disease. | |||
<br>In the United States alone, <i>Salmonella</i> is responsible for 1.4 million infections, 15,000 hospitalization, and 400 deaths per year [7]. These statistics are attributed to all serovars of <i>Salmonella enterica</i> not solely <i>Enteritidis</i>. | |||
<br><br><br> | |||
==Pathogenesis== | |||
<br>[[Image:salmonellaC_icon.jpg|thumb|800px|left|[Figure 2]Salmonella enterica serovar Enteritidis.[http://www.eiu.org/experiments/dispersion/images/salmonellaC_icon.jpg].]] | |||
<br><i>Salmonella Enteritidis</i> is a rod-shaped, gram-negative, proteobacteria that is non-motile [Figure 4]. <i>Salmonella Enteritidis</i> frequently contain plasmids that range from 55-60 kbp [8]. These facultative anaerobes are well adapted to survive in condition with our without oxygen, allowing them to live in diverse environments [9]. However, <i>Salmonella enteritidis</i> are usually found within animal hosts where they can be transmitted easily through food processing or fecal matter in areas with poor sanitation. | |||
<br>There are 27 phage types differentiated within the serovar Enteritidis [10]. These bacteriophages are viruses that infect the bacteria. The current dominant phage type worldwide is PT4 [Figure 3]. However PT8 is also commonly found in the US and UK. PT4, PT8, and PT13a comprise the majority of Enteritidis infections caused worldwide [11]. Different strains of the serovar Enteritidis are resistant to various phages. | |||
<br>The disease that <i>Salmonella Enteritidis</i> causes is known as a zoonotic disease, meaning that it transmits from animal to human. <i>Salmonella Enteritidis</i> begins when a person ingests a food item infected with a high concentration of the bacteria. In healthy adults, the acidity of the stomach can kill the bacteria if they are present in a low concentration. However, if a large amount of the bacteria are ingested, they have a higher probability of surviving and reproducing [12]. Once in the intestines, the bacteria invade the cells lining the intestine. The initial presence of bacteria ruffles the host cell's membrane creating an efficient route for bacteria to obtain necessary macromolecules. This is known as macropinocytosis. Once established in the intestine, the bacteria's virulence factors go to work. An enterotoxin results in the release of fluids from the cell into the lumen. This factor is responsible for the diarrhea and vomiting symptoms. Next, the endotoxin results in the release of endogenous pyrogens from the host cell, causing a fever in the victim. Lastly, the cytotoxin is responsible for the disintegration of the cytoplasm. It accomplishes this by inhibiting protein synthesis and causes calcium ions to rush in [13]. After the virulence factors have done their duty, the bacteria can move to the liver or spleen, where they are able to replicate. After replication, they can migrate back to the intestines where they can be expelled and transmitted to new hosts. | |||
<br><i>S. Enteritidis</i> require glucose to survive and uses mixed acid heterofermentation of glucose to produce energy. This method of metabolism releases carbon dioxide and hydrogen gas as a bi-product that then builds up in the victim’s intestinal system causing irritation. Acids such as acetate, formate, succinate, and lactate are also products of this fermentation. The bacteria reproduce extremely quickly causing illness due to the sheer number of foreign bacteria present. At the peak of infection, there can be up to one billion <i>Salmonella</i> bacteria present per gram of feces [14]. | |||
<br><br><br> | |||
==Research: A Case Study== | |||
<br>[[Image:phage.jpg|thumb|300px|left|[Figure 3]Salmonella enterica serovar Enteritidis Phage 4 genome.[http://www.ncbi.nlm.nih.gov/core/lw/2.0/html/tileshop_pmc/tileshop_pmc_inline.html?title=An%20external%20file%20that%20holds%20a%20picture%2C%20illustration%2C%20etc.%0AObject%20name%20is%201624fig2.jpg%20%5BObject%20name%20is%201624fig2.jpg%5D&p=PMC3&id=2556274_1624fig2.jpg].]] | |||
<br>One method for studying various strains of <i>Salmonella</i> is the use of pulsed-field gel electrophoresis clusters. In a specific case study carried out in Minnesota, the outbreak statistics were easily traced due to the fact that <i>Salmonella</i> infections are required to be reported by state law. This study took a look at confirmed <i>Salmonella enterica</i> infections in the state of Minnesota between January 1, 2001 through December 31, 2007. They examined the clusters of reported cases individually to determine more specifically the serovars of <i>Salmonella enterica</i>. A cluster was defined as two or more cases of <i>Salmonella</i> in different households with isolates from the same serovar and pulse-field gel electrophoresis subtype. In addition, the specimen had to be within two weeks of the onset of illness [15]. | |||
<br>Gel electrophoresis is a process by which fragments of DNA are separated using electrical current. The negative charge of DNA is attracted to the positive charge at the opposite end of the gel and therefore DNA moves across the gel. DNA fragments of smaller size are able to move further along the gel, thus separating the fragments by size. The problem with gel electrophoresis is that sometimes large fragments of DNA are not fully separated. In 1984, two scientists by the names of Schwartz and Cantor came up with the technique of pulse-field gel electrophoresis (PFGE). This technique involves intermittently changing the direction of the current to ensure that similar sizes are adequately separated. Therefore, this method is very important in distinguishing subtypes in the same serovars of bacteria. Due to their almost identical genetic makeup, PFGE allows for an even more specific analyzation of the genome than PCR alone [16]. | |||
<br>In addition to subtyping species of bacteria, phage typing can also be performed. Scientists phage type <i>S. Enteritidis</i> by grouping strains based on a set of 16 bacteriophages creating a lytic pattern. They will then compare DNA of the entire genome, partial genome, or a signature genetic marker of the phage type [17]. | |||
<br>The results of the study showed that 194 serovars of <i>Salmonella</i> were found in the reported cases. Out of these 194 cases, 20.5% were of the <i>Salmonella Enteritidis</i> serovar. However, only 13% of the identified clusters in all cases were solved. They concluded that the cluster size and density were important in predicting whether or not the case could be solved. The larger the cluster size and the higher the density, the more likely the case was to be solved. Because <i>Salmonella Enteritidis</i> made up a significant portion of the reported cases, this strain was more likely to be identified. The method of PFGE used to identify subtypes is also more useful when analyzing common serovars. This causes a slight bias in identifying specific serovars of bacteria. However, this study does reaffirm that <i>Salmonella Enteritidis</i> is one of the major serovars found in cases of food poisoning. | |||
<br><br><br> | <br><br><br> | ||
==Symptoms and Diagnosis== | |||
<br>The most common symptoms of <i>Salmonella Enteritidis</i> include fever, diarrhea, vomiting, abdominal cramps, muscle aches, and headache. These symptoms generally occur between 12-72 hours after the bacteria has been ingested and last anywhere from 4-7 days. Healthy individuals can usually rid themselves of the bacteria on their own; however, children, elderly people, and those with compromised immune systems may require additional treatment [18]. Additional treatment may take longer than 7 days to rid the body of bacteria. Those that are prescribed antibiotics generally take them for at least 10 days, even if their symptoms go away. Finishing the antibiotic prescription ensures that all <i>Salmonella</i> are killed in the patient's system. | |||
<br>Although these symptoms are an easy way to detect food poisoning, there are also clinical ways of testing for the presence of bacteria and more specifically <i>Salmonella Enteritidis</i>. A complete blood count can be done to look for anemia. Anemia, or a low iron content in red blood cells, can be a sign of bacterial infection. Iron is needed as an oxygen carrier in the blood; however, bacteria also require iron as a nutrient and can therefore deplete the blood's iron concentration. A low white blood count can also be a sign of bacterial infection. The liver function may be slightly elevated due to the fact that the bacteria are reproducing in the liver or spleen [19]. More specifically, a stool culture may be done to determine if the bacterial infection is in fact <i>Salmonella Enteritidis</i>. Once isolated from the stool, PCR amplification can be used to determine the strain present. Serotyping can be performed in order to identify the serovar of <i>Salmonella enterica</i>. This method involves immunological tests to detect specific bacterial proteins present in the sample [20]. | |||
== | |||
==Prevention and Treatment== | |||
<br>The most effective way to prevent <i>Salmonella</i> infections is to thoroughly cook all meats and eggs. The United States Department of Agriculture has also published guidelines to prevention. Their website gives a list of the proper cooking temperatures for all types of meat. They also include a list of proper sanitation when handling raw meat. These include washing your hands and cooking surfaces often as well as washing hands, cooking utensils, and cooking surfaces with hot, soapy water before handling any food. Because red meat, poultry, and seafood are more susceptible to carrying <i>Salmonella</i>, they also recommend separating these foods from other food in your refrigerator. Proper refrigeration is very important both before and after cooking. This is a huge problem in developing countries where raw meat can sit at room temperature for hours or even days before being cooked. This allows the bacteria to multiply, thus making it harder to ensure that every single bacterium is killed during the cooking process. Quickly cooling food right after cooking is also important. While hot temperatures kill the bacteria, warm temperatures provide the optimal environment for those bacteria that survived to multiply. Thus, switching from one extreme environment to another is the best way to ensure the lowest concentration of bacteria present in the food. All of these are important steps in preventing <i>Salmonella</i> outbreaks [4].<br> | |||
<br>As of right now, no vaccine exists for the the prevention of <i>Salmonella</i> in humans. However, healthy individuals usually can rid themselves of <i>Salmonella enteritidis</i> on their own by flushing it out of their system. Because the bacteria cause gastrointestinal inflammation, one of the most common symptoms is diarrhea. <i>Salmonella enteritidis</i> is therefore excreted in the feces. Once all of the bacteria are excreted, the gastrointestinal tract will return to normal and symptoms will subside. | |||
<br>For those cases that require medical treatment, typically elderly people or children, the most common method is through the use of antibiotics. There are a multitude of antibiotics available for treatment of <i>Salmonella Enteritidis</i> such as ampicillin, amoxicillin, ciprofloxacin, or trimethoprim/sulfamethozazole (TMP-SMX). However, the rising use of antibiotics to promote the growth of feed chickens has caused a rise in antibiotic resistance. Therefore, a combination of antibiotics may be required to completely rid the body of the bacteria [13]. Once the strain of <i>Salmonella</i> is isolated, doctors can narrow down the treatment to the most effective antibiotic. Different strains have different levels of sensitivity to each of the antibiotics; therefore, not all <i>Salmonella</i> infections will be treated with the same drug. | |||
<br>Because <i>S. Enteritidis</i> causes gastroenteritis, or intestinal irritation, one of the body's most effective methods to ridding the body of bacteria is through feces. While diarrhea rids the body of the bacteria, it also causes the problem of excessive water loss. Oral hydration is effective, but it may not be enough. Some patients require intravenous hydration in order to ensure that the organs are getting enough water. | |||
<br> | <br> | ||
<br><br><br> | ==Production of <i>Salmonella enteritidis</i> in Contaminated Eggs== | ||
<br>An experiment performed in the late 1980s examined the egg production and frequency of <i>Salmonella enteritidis</i> infection in chickens that were experimentally infected with a strain of <Salmonella enteritidis</i> (SE6). Richard K. Gast and C.W. Beard decided to infect chickens with this strain by either oral inoculation or contact transmission. Hens that were orally inoculated were given a dose of an overnight tryptone soya broth culture that contained the phage type 13a strain of <i>S. enteritidis</i>. Hens that were tested for pathogenesis through contact transmission shared feeding troughs and drinking cups with inoculated birds in adjacent cages. Lastly, they inoculated a third group of hens with a sterile broth to serve as a control. They then took cloacal swab samples at weekly intervals to keep track of the persistance of <i>S. enteritidis</i> in the intestinal tract. They took samples of intact eggs from each of the three groups everyday and tested for the presence of <i>S. enteritidis</i> both on the shell surface and inside the egg's contents. | |||
[[Image:SE11.jpeg|thumb|500px|right|[Figure 4]Presence of <i>S. enteritidis</i> on eggshells produced by experimentally infected hens.[http://www.jstor.org/stable/pdfplus/1591433.pdf?acceptTC=true].]] | |||
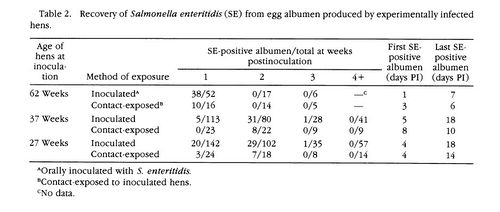
<br>The results showed that 91% of the orally inoculated 62-week-old hens tested positive for the presence of <i>S. enteritidis</i> in their intestinal tract. None of the control hens tested positive for <i>S. enteritidis</i>. <i>S. enteritidis</i> was found in the intestinal tracts of contact-exposed hens, but at a significantly lower frequency than the hens that were given an oral inoculation. The presence of SE6 on the eggshells occured on 53% of the eggs laid by orally inoculated 62-week-old hens. The presence of SE6 on the eggshells of contact-exposed hens was only 14%, significantly lower than that of orally inocualted hens [Figure 4]. Both of these samples were taken one week post-inoculation. SE6 was isolated from the albumen of 62.5% of the eggs that were laid by 62-week-old hens, 1 week post-inoculation, that were exposed to <i>S. enteritidid</i> through contact transmission. <i>S. enteritidis</i> was not present in the egg albumen of any of the control hens. SE6 was found significantly more in the albumen of eggs laid by 62-week-old hens, post-inoculation, that received the oral inoculation of bacteria. 73% of these eggs contained <i>S. enteritidis</i> in their albumen [Figure 5]. The presence of <i>S. enteritidis</i> in albumen from 27 and 37-week-old hens did not have a significant difference between eggs laid by orally inoculated hens and contact-exposed hens. Lastly, egg yolks were tested for the presence of <i>S. enteritidis</i>. The frequency of bacterial isolation from egg yolk in orally inoculated hens and contact-exposed hens did not differ significantly. | |||
[[Image:SE22.jpeg|thumb|500px|right|[Figure 5]Presence of <i>S. enteritidis</i> in albumen of eggs produced by experimentally infected hens.[http://www.jstor.org/stable/pdfplus/1591433.pdf?acceptTC=true].]] | |||
<br>These results show that orally inoculated hens were more likely to have intestinal colonization of <i>S. enteritidis</i> than contact-exposed hens. However, some of the contact-exposed hens that did not have <i>S. enteritidis</i> present in their intestinal tract still produced eggs with contaminated contents. This shows that evidently the presence of SE6 in the intestinal tract is not required in order to produce infected eggs. Although the mechanism of transmission from hen to egg before the egg is laid is not well understood, this experiment provides a little more insight into understanding <i>S. enteritidis</i> infections in our poultry. It is thought that the presence of <i>S. enteritidis</i> on eggshell comes from the hen's fecal matter containing the bacteria. When the hens lay the egg, fecal matter can get onto the eggshell and thus contaminate the egg. However, this experiment provides more proof that the egg's contents can be contaminated before the eggs are even laid. Because <i>S. enteritidis</i> was isolated at a consistently higher percentage of samples of egg contents than from shells, it appears that not all eggs infected on the inside are also infected on the outside. In order for bacterial presence on the eggshell to be the only method of transmission into the egg's contents, all eggs contaminated on the inside would also have to test positive for <i>S. enteritidis</i> on the outside[21]. Also, because contaminated eggshells typically become contaminated by feces, it would make sense that eggs laid 2,3, and 4 weeks post-inoculation are unlikely to have SE6 present on their eggshells. Because SE6 is flushed out through feces, healthy hens could rid themselves of all bacteria within a week. Thus eggs laid later than one week after infection would not come into contact with contaminated feces. | |||
<br>Transovarian transmission of <i>Salmonella enteritidis</i> is an area that even in 2011 is still not well understood. This makes <i>Salmonella</i> infections hard to completely wipe out, even in developed countries such as the United States and the UK. A disinfected, intact egg can contain these microscopic bacteria that can leave one bedridden for days. No matter how many sterilization precautions the food industry takes with both the laying hens and the eggs, there is still a possibility of contracting an infection. Further research into the mechanism of transovarian transmission is key to preventing foodborne illness caused by <i>Salmonella enteritidis</i>. | |||
==<i>Salmonella Enteritidis</i> in Our Poultry== | |||
<br>If <i>Salmonella Enteritidis</i> can infect the hen's ovaries and thus infect the egg before the shell forms, then how in the world do we ensure consumers that these eggs are safe to eat? Unfortunately, the mechanism by which the bacteria get into the forming egg is not well understood. However, an experiment performed by E.S. Soliman et al. studied the effect of stressors on the chickens that could cause higher rates of colonization within the chickens. | |||
<br>From previous studies, E.S. Soliman et al. found that environmental stress was a contributing factors that "may induce colonization of food animals by enteric pathogens, facilitate horizontal transmission of pathogens between animals, increase pathogen shedding and contribute in carcass contamination during processing". Chickens have quite a journey from being born into a commercial poultry farm to being wrapped in cellophane at the grocery store. The two factors that E.S. Soliman et al. studied were the effects of temperature fluctuations and fasting. Chickens deal with temperature fluctuations in seasonal environments as well as transportation. Chickens are also subjected to fasting before slaughter. The purpose of this is to have the least amount of food in the intestines as possible in order to minimize the risk of contamination via carcasses during processing. | |||
<br>This experiment first examined the effect of heat stress on the colonization of <i>Salmonella Enteritidis</i> in ileum tissue. This is a portion of tissue that lines the intestines. Chickens living in temperate conditions were used as a control. Results showed that the ileum tissue of heat-stressed chickens was a factor in causing increased susceptibility to <i>Salmonella Enteritidis</i> colonization. They proposed that this could be due to the fact that heat stress could damage the mucosal structure within the intestines. This mucous membrane protects the gastrointestinal tract from foreign bodies and aides in digestion. Also, the researchers proposed that heat shock proteins may act as receptors for pathogen binding. These receptors would be located on the epithelial surface, which is quite accessible to invading bacteria. | |||
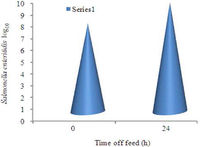
<br>In addition, feed was withdrawn from one sample of chickens, while chickens given food served as the control. Results showed that intestinal tissue collected from fasting chickens were also more susceptible to pathogen attachment. Fasting caused a similar morphological effect in the intestinal tract to the heat-stress. Study showed that there was a linear reduction of mucosal lining over the 24 hour fasting period [Figure 6]. This, again, reduces the protection of the gastrointestinal tract. | |||
[[Image:feed.jpg|thumb|200px|right| [Figure 6]Effect of 24 hour feed withdrawal on ileal susceptibility to <i>Salmonella enteritidis</i> attachment.[http://www.scipub.org/fulltext/AJAV/AJAV4342-48.pdf].]] | |||
< | The authors summed up their study stating, "Therefore, stress-induced the integrity of the gut epithelium reduces the innate protective mechanisms and may increase the potential for pathogens such as <i>Salmonella</i> to bind to and colonize the intestinal epithelium. Such colonization in poultry will increase the risk of carcass contamination during processing and will increase the potential for <i>Salmonella</i> to translocate to the reproductive tract, where it can contaminate eggs during formation" [22]. | ||
<br>It is important to note that regardless of the fact that the humane treatment of chickens and other feed animals is morally ethical, it is also more sanitary. Further study should be done in order to truly determine whether making chickens fast is beneficial to reducing contamination. This and other studies have shown that heat-stress is harmful to chickens. Therefore, it is important to ensure that chickens are grown in appropriate environments to promote healthy growth, which in turn makes our food safer to eat. America has recently gone "organic" consuming many natural foods and free-range meats. Free-range animals are those that are raised in a natural environment, feeding when they like, inhabiting where they are comfortable, and not given artificial growth hormones to increase muscle size. These new methods of raising meat are looking more and more sanitary as new research comes to light. | |||
<br> | |||
==Conclusion== | ==Conclusion== | ||
<br> | <br>While <i>Salmonella</i> rarely causes death in areas where sanitary precautions are taken, it is unsettling to know that <i>Salmonella Enteritidis</i> can silently infect eggs. This characteristic makes it increasingly common as a foodborne pathogen in developed countries. The exact mechanism of this infection is not well understood and is an important area of research for Departments of Health around the globe. While we rarely think of people dying from food poisoning, bacterial infections can be quite dangerous. Because of the world's ever increasing population size, researchers are always looking for new methods to produce food in larger quantities and in shorter periods of time. While our meat industry fills animals with antibiotics and growth hormones, they can unknowingly be contributing to the growth and antibiotic resistance to pathogenic bacteria. As bacteria become resistant to more and more antibiotics, scientists must find alternative methods to combat the rapid reproduction of these microbes. The battle between bacterial resistance and antibiotic production is a rising problem that will only get worse as bacteria continue to naturally select against antibiotics. Therefore, it is important to learn as much as possible about bacterial metabolism in order to keep bacterial populations under control.<br> | ||
==References== | ==References== | ||
[ | 5. [http://www.fsis.usda.gov/Fact_Sheets/Salmonella_Questions_&_Answers/index.asp USDA <i>Salmonella</i> Questions and Answers.] | ||
6.[http://www.medicinenet.com/script/main/art.asp?articlekey=22060 MedicineNet: <i>Salmonella Enteritidis</i> Infection (Egg-associated Salmonellosis)] | |||
7.[http://www.igs.umaryland.edu/research/infec/infectious_diseases_projects.php Fricke, Florian W. and Ravel, Jacques. Genome diversity among non-typhoidal Salmonella enterica. University of Maryland; Institute for Genome Sciences.] | |||
8.[http://www.biomedcentral.com/1471-2180/7/87 Olson et al. Limited genetic diversity in <i>Salmonella enterica</i> Serovar <i>Enteritidis</i> PT13. 2007. BMC Microbiology 7:87.] | |||
9. [http://www.news.cornell.edu/releases/jan98/DT104facts.html Cornell University: Salmonella fact sheet.] | |||
10. [http://jb.asm.org/cgi/content/short/187/18/6545 Porwollik, S. et al. Differences in Gene Content Between <i>Salmonella enterica</i> Serovar Enteritidis Isolates and Comparison to Closely Related Serovars Gallinarum and Dublin. 2005. Journal of Bacteriology Volume 187(18): 6545-6555.] | |||
11.[http://mic.sgmjournals.org/cgi/reprint/155/10/3200 Zhensheng, Pan et al. Identification of genetic and phenotypic differences associated with prevalent and non-prevalent <i>Salmonella</i> Enteritidis phage types: analysis of variation in amino acid transport. 2009. Microbiology 155:3200-3213.] | |||
12. [http://en.wikipedia.org/wiki/Salmonella Wikipedia: <i>Salmonella</i>.] | |||
13. [http://oscar.virginia.edu/asp/PublicAward.asp?AwardID=7962 Casanova, James E. Mechanism of Salmonella Invasion and Transmigration. Oscar research at UVA.] | |||
14. [http://www.medicinenet.com/salmonella/article.htm MedicineNet:<i>Salmonella</i> Poisoning.] | |||
15.[http://www.cdc.gov/eid/content/16/11/1678.htm Rounds, Joshua M. et al. <i>Salmonella enterica</i> Pulsed-Field Gel Electrophoresis Clusters, Minnesota, USA, 2001-2007. 2010. Emerging Infectious Diseases Journal, Volume 16, Number 11.] | |||
16. [http://www.pritzkerlaw.com/section-foodborne-illness/food-safety-lawyer/PFGE.html Pritzker Personal Injury and Wrongful Death Lawyer. How Does PFGE work?] | |||
17.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC270439/ Hickman-Brenner, F.W., Stubbs, A.D., and Farmer, J.J. Phage typing of Salmonella enteritidis in the United States. 1991. J. Clin Microbiology (12): 2817-2823.] | |||
18. [http://www.mayoclinic.com/health/salmonella/DS00926/DSECTION=symptoms Mayo Clinic: <i>Salmonella</i> infection.] | |||
19.[http://emedicine.medscape.com/article/785774-workup Medscape Reference: Salmonella Infection in Emergency Medicine Workup.] | |||
20. [http://www.wpro.who.int/wpsar/archives/Archive_2(1)2011_OR_Suhana+et+al.htm Solhan, Suhana et al. An outbreak of gastroenteritis caused by <i>Salmonella enterica</i> serotype Enteritidis traced to cream cakes. 2011. Western Pacific Surveillance and Response Journal Volume 2, Issue 1: 1-8.] | |||
21.[http://www.jstor.org/stable/pdfplus/1591433.pdf?acceptTC=true Gast, Richard K. and Beard, C.W. Production of Salmonella enteritidis-Contaminated Eggs by Experimentally Infected Hens. 1990. Avian Diseases Volume 34, No. 2: p.438-446.] | |||
22.[http://www.scipub.org/fulltext/AJAV/AJAV4342-48.pdf E.S. Soliman et al. Stressors Influence on <i>Salmonella enterica</i> Serovar <i>Enteritidis</i> Colonization in Broilers. 2009. American Journal of Veterinary Sciences 4 (3): 42-48.] | |||
Edited by student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl09.html BIOL 238 Microbiology], 2009, [http://www.kenyon.edu/index.xml Kenyon College]. | Edited by student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl09.html BIOL 238 Microbiology], 2009, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | ||
Latest revision as of 19:21, 12 May 2011

The bacteria Salmonella is commonly associated with food poisoning in countries all over the world. There are two species of Salmonella: S. enterica and S. bongori. However, the species that most people refer to when they talk about Salmonella is S. enterica. This species is divided into a subset comprising of serovars. In S. enterica alone, there are over 2,000 serovars of bacteria. Salmonella infections can originate from household pets containing the bacteria, particularly reptiles, improperly prepared meats and seafood, or the surfaces of raw eggs, fruits, or vegetables that have not been adequately disinfected. The most common cause of food poisoning is associated with the serovar S. Enteritidis. These outbreaks are attributed to contaminated poultry, chicken eggs, and products that contain eggs. Because of the high demand for poultry and poultry products, farms can become highly unsanitary [Figure 1]. For example, chicken farms contain thousands up on thousands of chickens all living in the same area. Dead chickens are left for days before they are removed from the others. If one chicken contains the Salmonella bacteria, the organism can spread rapidly through thousands of others. When these chickens are processed and sent to stores all over the country, a mass Salmonella outbreak can occur [5]. When humans contract this disease, they can expel some of the Salmonella bacteria in their feces. In areas with poor sanitation, these bacteria can get into the water system where they can infect another person or animal and begin a whole new cycle of transmission.
Poultry eggs can contain Salmonella bacteria both on the surface as well as inside. After hens lay eggs, they sit on top of them to keep them warm. While sitting, fecal matter can transfer from the chicken to the surface of the egg. If the chicken is infected with Salmonella, the eggs must be properly disinfected in order to prevent disease. This is a larger problem in developing countries rather than the US or UK. The more common method of transmittance in the US is attributed to eggs infected with Salmonella on the inside. Salmonella Enteritidis can infect the ovaries of a hen and thus contaminate the egg before the shell forms [6]. Because the bacterium silently infects the hen’s ovaries, the hen does not show any signs of an illness. Therefore, seemingly healthy hens transmit this disease.
In the United States alone, Salmonella is responsible for 1.4 million infections, 15,000 hospitalization, and 400 deaths per year [7]. These statistics are attributed to all serovars of Salmonella enterica not solely Enteritidis.
Pathogenesis
Salmonella Enteritidis is a rod-shaped, gram-negative, proteobacteria that is non-motile [Figure 4]. Salmonella Enteritidis frequently contain plasmids that range from 55-60 kbp [8]. These facultative anaerobes are well adapted to survive in condition with our without oxygen, allowing them to live in diverse environments [9]. However, Salmonella enteritidis are usually found within animal hosts where they can be transmitted easily through food processing or fecal matter in areas with poor sanitation.
There are 27 phage types differentiated within the serovar Enteritidis [10]. These bacteriophages are viruses that infect the bacteria. The current dominant phage type worldwide is PT4 [Figure 3]. However PT8 is also commonly found in the US and UK. PT4, PT8, and PT13a comprise the majority of Enteritidis infections caused worldwide [11]. Different strains of the serovar Enteritidis are resistant to various phages.
The disease that Salmonella Enteritidis causes is known as a zoonotic disease, meaning that it transmits from animal to human. Salmonella Enteritidis begins when a person ingests a food item infected with a high concentration of the bacteria. In healthy adults, the acidity of the stomach can kill the bacteria if they are present in a low concentration. However, if a large amount of the bacteria are ingested, they have a higher probability of surviving and reproducing [12]. Once in the intestines, the bacteria invade the cells lining the intestine. The initial presence of bacteria ruffles the host cell's membrane creating an efficient route for bacteria to obtain necessary macromolecules. This is known as macropinocytosis. Once established in the intestine, the bacteria's virulence factors go to work. An enterotoxin results in the release of fluids from the cell into the lumen. This factor is responsible for the diarrhea and vomiting symptoms. Next, the endotoxin results in the release of endogenous pyrogens from the host cell, causing a fever in the victim. Lastly, the cytotoxin is responsible for the disintegration of the cytoplasm. It accomplishes this by inhibiting protein synthesis and causes calcium ions to rush in [13]. After the virulence factors have done their duty, the bacteria can move to the liver or spleen, where they are able to replicate. After replication, they can migrate back to the intestines where they can be expelled and transmitted to new hosts.
S. Enteritidis require glucose to survive and uses mixed acid heterofermentation of glucose to produce energy. This method of metabolism releases carbon dioxide and hydrogen gas as a bi-product that then builds up in the victim’s intestinal system causing irritation. Acids such as acetate, formate, succinate, and lactate are also products of this fermentation. The bacteria reproduce extremely quickly causing illness due to the sheer number of foreign bacteria present. At the peak of infection, there can be up to one billion Salmonella bacteria present per gram of feces [14].
Research: A Case Study

One method for studying various strains of Salmonella is the use of pulsed-field gel electrophoresis clusters. In a specific case study carried out in Minnesota, the outbreak statistics were easily traced due to the fact that Salmonella infections are required to be reported by state law. This study took a look at confirmed Salmonella enterica infections in the state of Minnesota between January 1, 2001 through December 31, 2007. They examined the clusters of reported cases individually to determine more specifically the serovars of Salmonella enterica. A cluster was defined as two or more cases of Salmonella in different households with isolates from the same serovar and pulse-field gel electrophoresis subtype. In addition, the specimen had to be within two weeks of the onset of illness [15].
Gel electrophoresis is a process by which fragments of DNA are separated using electrical current. The negative charge of DNA is attracted to the positive charge at the opposite end of the gel and therefore DNA moves across the gel. DNA fragments of smaller size are able to move further along the gel, thus separating the fragments by size. The problem with gel electrophoresis is that sometimes large fragments of DNA are not fully separated. In 1984, two scientists by the names of Schwartz and Cantor came up with the technique of pulse-field gel electrophoresis (PFGE). This technique involves intermittently changing the direction of the current to ensure that similar sizes are adequately separated. Therefore, this method is very important in distinguishing subtypes in the same serovars of bacteria. Due to their almost identical genetic makeup, PFGE allows for an even more specific analyzation of the genome than PCR alone [16].
In addition to subtyping species of bacteria, phage typing can also be performed. Scientists phage type S. Enteritidis by grouping strains based on a set of 16 bacteriophages creating a lytic pattern. They will then compare DNA of the entire genome, partial genome, or a signature genetic marker of the phage type [17].
The results of the study showed that 194 serovars of Salmonella were found in the reported cases. Out of these 194 cases, 20.5% were of the Salmonella Enteritidis serovar. However, only 13% of the identified clusters in all cases were solved. They concluded that the cluster size and density were important in predicting whether or not the case could be solved. The larger the cluster size and the higher the density, the more likely the case was to be solved. Because Salmonella Enteritidis made up a significant portion of the reported cases, this strain was more likely to be identified. The method of PFGE used to identify subtypes is also more useful when analyzing common serovars. This causes a slight bias in identifying specific serovars of bacteria. However, this study does reaffirm that Salmonella Enteritidis is one of the major serovars found in cases of food poisoning.
Symptoms and Diagnosis
The most common symptoms of Salmonella Enteritidis include fever, diarrhea, vomiting, abdominal cramps, muscle aches, and headache. These symptoms generally occur between 12-72 hours after the bacteria has been ingested and last anywhere from 4-7 days. Healthy individuals can usually rid themselves of the bacteria on their own; however, children, elderly people, and those with compromised immune systems may require additional treatment [18]. Additional treatment may take longer than 7 days to rid the body of bacteria. Those that are prescribed antibiotics generally take them for at least 10 days, even if their symptoms go away. Finishing the antibiotic prescription ensures that all Salmonella are killed in the patient's system.
Although these symptoms are an easy way to detect food poisoning, there are also clinical ways of testing for the presence of bacteria and more specifically Salmonella Enteritidis. A complete blood count can be done to look for anemia. Anemia, or a low iron content in red blood cells, can be a sign of bacterial infection. Iron is needed as an oxygen carrier in the blood; however, bacteria also require iron as a nutrient and can therefore deplete the blood's iron concentration. A low white blood count can also be a sign of bacterial infection. The liver function may be slightly elevated due to the fact that the bacteria are reproducing in the liver or spleen [19]. More specifically, a stool culture may be done to determine if the bacterial infection is in fact Salmonella Enteritidis. Once isolated from the stool, PCR amplification can be used to determine the strain present. Serotyping can be performed in order to identify the serovar of Salmonella enterica. This method involves immunological tests to detect specific bacterial proteins present in the sample [20].
Prevention and Treatment
The most effective way to prevent Salmonella infections is to thoroughly cook all meats and eggs. The United States Department of Agriculture has also published guidelines to prevention. Their website gives a list of the proper cooking temperatures for all types of meat. They also include a list of proper sanitation when handling raw meat. These include washing your hands and cooking surfaces often as well as washing hands, cooking utensils, and cooking surfaces with hot, soapy water before handling any food. Because red meat, poultry, and seafood are more susceptible to carrying Salmonella, they also recommend separating these foods from other food in your refrigerator. Proper refrigeration is very important both before and after cooking. This is a huge problem in developing countries where raw meat can sit at room temperature for hours or even days before being cooked. This allows the bacteria to multiply, thus making it harder to ensure that every single bacterium is killed during the cooking process. Quickly cooling food right after cooking is also important. While hot temperatures kill the bacteria, warm temperatures provide the optimal environment for those bacteria that survived to multiply. Thus, switching from one extreme environment to another is the best way to ensure the lowest concentration of bacteria present in the food. All of these are important steps in preventing Salmonella outbreaks [4].
As of right now, no vaccine exists for the the prevention of Salmonella in humans. However, healthy individuals usually can rid themselves of Salmonella enteritidis on their own by flushing it out of their system. Because the bacteria cause gastrointestinal inflammation, one of the most common symptoms is diarrhea. Salmonella enteritidis is therefore excreted in the feces. Once all of the bacteria are excreted, the gastrointestinal tract will return to normal and symptoms will subside.
For those cases that require medical treatment, typically elderly people or children, the most common method is through the use of antibiotics. There are a multitude of antibiotics available for treatment of Salmonella Enteritidis such as ampicillin, amoxicillin, ciprofloxacin, or trimethoprim/sulfamethozazole (TMP-SMX). However, the rising use of antibiotics to promote the growth of feed chickens has caused a rise in antibiotic resistance. Therefore, a combination of antibiotics may be required to completely rid the body of the bacteria [13]. Once the strain of Salmonella is isolated, doctors can narrow down the treatment to the most effective antibiotic. Different strains have different levels of sensitivity to each of the antibiotics; therefore, not all Salmonella infections will be treated with the same drug.
Because S. Enteritidis causes gastroenteritis, or intestinal irritation, one of the body's most effective methods to ridding the body of bacteria is through feces. While diarrhea rids the body of the bacteria, it also causes the problem of excessive water loss. Oral hydration is effective, but it may not be enough. Some patients require intravenous hydration in order to ensure that the organs are getting enough water.
Production of Salmonella enteritidis in Contaminated Eggs
An experiment performed in the late 1980s examined the egg production and frequency of Salmonella enteritidis infection in chickens that were experimentally infected with a strain of <Salmonella enteritidis (SE6). Richard K. Gast and C.W. Beard decided to infect chickens with this strain by either oral inoculation or contact transmission. Hens that were orally inoculated were given a dose of an overnight tryptone soya broth culture that contained the phage type 13a strain of S. enteritidis. Hens that were tested for pathogenesis through contact transmission shared feeding troughs and drinking cups with inoculated birds in adjacent cages. Lastly, they inoculated a third group of hens with a sterile broth to serve as a control. They then took cloacal swab samples at weekly intervals to keep track of the persistance of S. enteritidis in the intestinal tract. They took samples of intact eggs from each of the three groups everyday and tested for the presence of S. enteritidis both on the shell surface and inside the egg's contents.
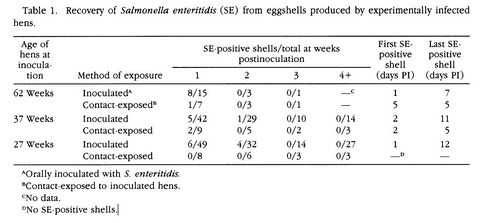
The results showed that 91% of the orally inoculated 62-week-old hens tested positive for the presence of S. enteritidis in their intestinal tract. None of the control hens tested positive for S. enteritidis. S. enteritidis was found in the intestinal tracts of contact-exposed hens, but at a significantly lower frequency than the hens that were given an oral inoculation. The presence of SE6 on the eggshells occured on 53% of the eggs laid by orally inoculated 62-week-old hens. The presence of SE6 on the eggshells of contact-exposed hens was only 14%, significantly lower than that of orally inocualted hens [Figure 4]. Both of these samples were taken one week post-inoculation. SE6 was isolated from the albumen of 62.5% of the eggs that were laid by 62-week-old hens, 1 week post-inoculation, that were exposed to S. enteritidid through contact transmission. S. enteritidis was not present in the egg albumen of any of the control hens. SE6 was found significantly more in the albumen of eggs laid by 62-week-old hens, post-inoculation, that received the oral inoculation of bacteria. 73% of these eggs contained S. enteritidis in their albumen [Figure 5]. The presence of S. enteritidis in albumen from 27 and 37-week-old hens did not have a significant difference between eggs laid by orally inoculated hens and contact-exposed hens. Lastly, egg yolks were tested for the presence of S. enteritidis. The frequency of bacterial isolation from egg yolk in orally inoculated hens and contact-exposed hens did not differ significantly.
These results show that orally inoculated hens were more likely to have intestinal colonization of S. enteritidis than contact-exposed hens. However, some of the contact-exposed hens that did not have S. enteritidis present in their intestinal tract still produced eggs with contaminated contents. This shows that evidently the presence of SE6 in the intestinal tract is not required in order to produce infected eggs. Although the mechanism of transmission from hen to egg before the egg is laid is not well understood, this experiment provides a little more insight into understanding S. enteritidis infections in our poultry. It is thought that the presence of S. enteritidis on eggshell comes from the hen's fecal matter containing the bacteria. When the hens lay the egg, fecal matter can get onto the eggshell and thus contaminate the egg. However, this experiment provides more proof that the egg's contents can be contaminated before the eggs are even laid. Because S. enteritidis was isolated at a consistently higher percentage of samples of egg contents than from shells, it appears that not all eggs infected on the inside are also infected on the outside. In order for bacterial presence on the eggshell to be the only method of transmission into the egg's contents, all eggs contaminated on the inside would also have to test positive for S. enteritidis on the outside[21]. Also, because contaminated eggshells typically become contaminated by feces, it would make sense that eggs laid 2,3, and 4 weeks post-inoculation are unlikely to have SE6 present on their eggshells. Because SE6 is flushed out through feces, healthy hens could rid themselves of all bacteria within a week. Thus eggs laid later than one week after infection would not come into contact with contaminated feces.
Transovarian transmission of Salmonella enteritidis is an area that even in 2011 is still not well understood. This makes Salmonella infections hard to completely wipe out, even in developed countries such as the United States and the UK. A disinfected, intact egg can contain these microscopic bacteria that can leave one bedridden for days. No matter how many sterilization precautions the food industry takes with both the laying hens and the eggs, there is still a possibility of contracting an infection. Further research into the mechanism of transovarian transmission is key to preventing foodborne illness caused by Salmonella enteritidis.
Salmonella Enteritidis in Our Poultry
If Salmonella Enteritidis can infect the hen's ovaries and thus infect the egg before the shell forms, then how in the world do we ensure consumers that these eggs are safe to eat? Unfortunately, the mechanism by which the bacteria get into the forming egg is not well understood. However, an experiment performed by E.S. Soliman et al. studied the effect of stressors on the chickens that could cause higher rates of colonization within the chickens.
From previous studies, E.S. Soliman et al. found that environmental stress was a contributing factors that "may induce colonization of food animals by enteric pathogens, facilitate horizontal transmission of pathogens between animals, increase pathogen shedding and contribute in carcass contamination during processing". Chickens have quite a journey from being born into a commercial poultry farm to being wrapped in cellophane at the grocery store. The two factors that E.S. Soliman et al. studied were the effects of temperature fluctuations and fasting. Chickens deal with temperature fluctuations in seasonal environments as well as transportation. Chickens are also subjected to fasting before slaughter. The purpose of this is to have the least amount of food in the intestines as possible in order to minimize the risk of contamination via carcasses during processing.
This experiment first examined the effect of heat stress on the colonization of Salmonella Enteritidis in ileum tissue. This is a portion of tissue that lines the intestines. Chickens living in temperate conditions were used as a control. Results showed that the ileum tissue of heat-stressed chickens was a factor in causing increased susceptibility to Salmonella Enteritidis colonization. They proposed that this could be due to the fact that heat stress could damage the mucosal structure within the intestines. This mucous membrane protects the gastrointestinal tract from foreign bodies and aides in digestion. Also, the researchers proposed that heat shock proteins may act as receptors for pathogen binding. These receptors would be located on the epithelial surface, which is quite accessible to invading bacteria.
In addition, feed was withdrawn from one sample of chickens, while chickens given food served as the control. Results showed that intestinal tissue collected from fasting chickens were also more susceptible to pathogen attachment. Fasting caused a similar morphological effect in the intestinal tract to the heat-stress. Study showed that there was a linear reduction of mucosal lining over the 24 hour fasting period [Figure 6]. This, again, reduces the protection of the gastrointestinal tract.
The authors summed up their study stating, "Therefore, stress-induced the integrity of the gut epithelium reduces the innate protective mechanisms and may increase the potential for pathogens such as Salmonella to bind to and colonize the intestinal epithelium. Such colonization in poultry will increase the risk of carcass contamination during processing and will increase the potential for Salmonella to translocate to the reproductive tract, where it can contaminate eggs during formation" [22].
It is important to note that regardless of the fact that the humane treatment of chickens and other feed animals is morally ethical, it is also more sanitary. Further study should be done in order to truly determine whether making chickens fast is beneficial to reducing contamination. This and other studies have shown that heat-stress is harmful to chickens. Therefore, it is important to ensure that chickens are grown in appropriate environments to promote healthy growth, which in turn makes our food safer to eat. America has recently gone "organic" consuming many natural foods and free-range meats. Free-range animals are those that are raised in a natural environment, feeding when they like, inhabiting where they are comfortable, and not given artificial growth hormones to increase muscle size. These new methods of raising meat are looking more and more sanitary as new research comes to light.
Conclusion
While Salmonella rarely causes death in areas where sanitary precautions are taken, it is unsettling to know that Salmonella Enteritidis can silently infect eggs. This characteristic makes it increasingly common as a foodborne pathogen in developed countries. The exact mechanism of this infection is not well understood and is an important area of research for Departments of Health around the globe. While we rarely think of people dying from food poisoning, bacterial infections can be quite dangerous. Because of the world's ever increasing population size, researchers are always looking for new methods to produce food in larger quantities and in shorter periods of time. While our meat industry fills animals with antibiotics and growth hormones, they can unknowingly be contributing to the growth and antibiotic resistance to pathogenic bacteria. As bacteria become resistant to more and more antibiotics, scientists must find alternative methods to combat the rapid reproduction of these microbes. The battle between bacterial resistance and antibiotic production is a rising problem that will only get worse as bacteria continue to naturally select against antibiotics. Therefore, it is important to learn as much as possible about bacterial metabolism in order to keep bacterial populations under control.
References
5. USDA Salmonella Questions and Answers.
6.MedicineNet: Salmonella Enteritidis Infection (Egg-associated Salmonellosis)
9. Cornell University: Salmonella fact sheet.
13. Casanova, James E. Mechanism of Salmonella Invasion and Transmigration. Oscar research at UVA.
14. MedicineNet:Salmonella Poisoning.
16. Pritzker Personal Injury and Wrongful Death Lawyer. How Does PFGE work?
18. Mayo Clinic: Salmonella infection.
19.Medscape Reference: Salmonella Infection in Emergency Medicine Workup.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
