Bordetella parapertussis: Difference between revisions
| (60 intermediate revisions by 2 users not shown) | |||
| Line 19: | Line 19: | ||
==Description and Significance== | ==Description and Significance== | ||
<i>Bordetella parapertussis</i> is a Gram-negative, non-motile coccobacillus. It is an obligate aerobe, and inhabits the ciliated upper respiratory epithelial cells of its hosts. <i>Bordetella parapertussis</i> has two hosts, sheep and humans. The two hosts possess two different strains <i>B. parapertussis (ov)</i> infecting sheep and <i>B. parapertussis (hu)</i> infecting humans. These two strains are completely isolated from each other and are genetically distinct, with cross-contamination being extremely rare. Their genetically distinct features indicate independent evolution from a common ancestor, <i>B. bronchiseptica</i>. [3] | |||
<i>Bordetella parapertussis</i> causes a highly infectious upper respiratory tract infection characterized by pertussis, an intense episode of coughing, along with symptoms such as whooping, paroxysm, apnea, vomiting, encephalopathy, and cyanosis. [2] B. parapertussis is most severe in young infants less than six months of age. [4] <i>Bordetella parapertussis</i> usually produces a milder form of whooping cough compared to <i>Bordetella pertussis</i>, the main cause of whooping cough worldwide, however, the epidemiology of the two are not easily distinguished. [2] B. parapertussis is estimated to make up 5-30% of whooping cough cases, particularly in regions with acellular pertussis immunization programs (ACV vaccinations). [4] | |||
The ACV vaccine targets the virulence factors of B. <i>pertussis</i>, which imposes selective pressure on B. <i>parapertussis</i>. B. <i>parapertussis</i> shares some, but not all of the virulence factors with B. <i>pertussis</i>. Therefore, rates of B. <i>parapertussis</i> cases are increasing worldwide because B. <i>parapertussis</i> can evade the ACV vaccine. Additionally, B. <i>parapertussis</i> strains with mutations that eliminate virulence factors, such the increasingly common PRN-negative B. <i>parapertussis</i> infecting humans, pose the greatest risk of becoming endemic due to the fact that the ACV vaccine is unable to use virulence factor mediated phagocytosis. [5] Given its highly infectious spread through respiratory droplets and its ability to evade current vaccines, ongoing research and surveillance efforts to understand <i>B. parapertussis</i> pathogenesis is crucial for disease management and vaccination strategies. | |||
==Genome Structure== | ==Genome Structure== | ||
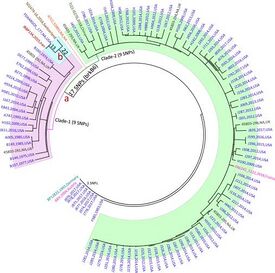
[[File:GCircle.jpeg|275px|thumb|right|Phylogenetic relationships of Iranian ''B. parapertussis'' isolate (IRBP134) with global isolates.[4]]] | |||
<i>Bordetella parapertussis</i> possesses a single circular chromosome, with a genome size of approximately 4.7 million base pairs (Mbp) and a G+C content of 65%. [4] Approximately 3,000 genes are shared between <i>B. pertussis</i>, <i>B. bronchiseptica</i>, and <i>B. parapertussis</i>. Both <i>B. parapertussis</i> and <i>B. pertussis</i> evolved from <i>B. bronchiseptica</i>. Only 50 genes are unique to <i>B. parapertussis</i>. [3] <i>B. parapertussis</i> shares a common ancestor with <i>B. pertussis</i>, the main cause of whooping cough worldwide, and has two distinct lineages from which it evolved independently from <i>B. bronchiseptica</i>. | |||
One strain of <i>B. parapertussis</i> causes a milder form of whooping cough in humans, <i>B. parapertussis (hu)</i>, and the other infects sheep, <i>B. parapertussis (ov)</i>. Both strains are almost entirely isolated from each other and there is little to no genetic exchange. <i>B. parapertussis (hu)</i> possesses 217 pseudogenes, has only one circular chromosome, and is more genetically uniform compared to <i>B. parapertussis (ov)</i>. <i>B. parapertussis (ov)</i> possesses 389 pseudogenes, is more genetically diverse, and has an additional plasmid that is 12 kilobases (Kb). [5] Unlike <i>B. pertussis</i>, <i>B. parapertussis</i> does not produce the pertussis toxin that makes <i>B. pertussis</i> so severe. Additionally, <i>B. parapertussis</i> also possesses a pseudogene for flagella, making it nonmotile, unlike <i>B. bronchiseptica</i>. [4] | |||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
[[File:LifeC.png|150px|thumb|left|''B. parapertussis'' life cycle [15]]] | |||
<i>Bordetella parapertussis</i> is a Gram-negative, non-motile, coccobacillus that is about 0.5-1.0 μm in length. [3] It can occur either singularly or in pairs. Therefore, it possesses a thin murein layer and a surrounding outer layer of lipopolysaccharide and phospholipids. It also produces a capsule. It is an obligate aerobe and a chemoorganoheterotroph. The primary source of carbon for <i>Bordetella parapertussis</i> is glutamate. It does not metabolize sugars because it lacks the genes for glucokinase, phosphofructokinase, and fructose-1,6-bisphosphate. It also does not perform fermentation or the TCA cycle. B. parapertussis also does not use cytochrome c oxidase during respiratory metabolism. [3] Additionally, because <i>Bordetella parapertussis</i> requires iron to survive, it performs hemolysis to absorb iron through siderophores. [6] | |||
<i>Bordetella parapertussis</i> is transmitted through respiratory droplets and cannot survive in the outside environment. Upon entering the host, <i>B. parapertussis</i> encounters its optimal growth temperature (37°C), which activates the BvgA/S pathway. The BvgA/S pathway initiates the transcription of virulence-activating genes, which signals host colonization of the ciliated respiratory epithelial cells. <i>B. parapertussis</i> possesses many virulence factors that sustain its growth and reproduction. [7] | |||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
[[File:Ptact.png|200px|thumb|right|Pertactin [7]]] | |||
Filamentous Hemagglutinin (FMA): | |||
One of the most notable virulence factors is Filamentous Hemagglutinin (FHA). FHA is a long, rod-shaped protein that is both a surface antigen and a secreted protein. As a surface antigen, it binds to ciliated respiratory epithelial cells and allows tracheal colonization. FHA promotes Interleukin 10, a cytokine with anti-inflammatory properties, [8] and reduces Interleukin 12, which promotes both the innate and adaptive immune response. [9] When FHA is secreted it binds to monocytes where it inhibits antigen-dependent CD4+ T cell proliferation and generates apoptosis. [=====16] CD4+ T cells rely on antigen presenting monocytes to activate. CD4+ T cells are responsible for producing cytokines, which help activate the specific immune response, and recruit Macrophages, cytotoxic T cells, and B cells. Suppression of the specific immune response limits destruction of the pathogen by macrophages and cytotoxic T cells and the production of neutralizing antibodies by B cells. [10] | |||
Pertactin (PRN): | |||
Another important virulence factor is Pertactin (PRN). It is an autotransporter responsible for adhesion to the ciliated respiratory cells. PRN is comprised of 16 right-handed parallel β-helixes. It is the largest β-helix structure ever recorded. The ACV vaccine targets PRN for phagocytosis through a cascade of immunological responses mediated by C1q. When antibodies bind to the PRN antigen, they arrange on the bacterial surface in a conformation favorable for C1q binding. C1q binding activates a cascade of synergistic activities: the formation of a Membrane Attack Complex (MAC) which forms pores in the bacterial surface leading to osmolysis, the depositing of C3b factors which act as opsonins (or tags for immune system recognition), and the binding of FcR (fragment crystallizable region) to PRNs to activate phagocytosis. [7] PRN is an important target for the ACV vaccine. However, PRN-negative ''B. parapertussis'' is becoming increasing common worldwide, likely due to the selective pressures of the ACV vaccine. PRN-negative ''B. parapertussis'' were shown to have either one or both nonsense mutations: either a deletion of an adenine in region I or a guanine deletion in region II of the PRN gene. [4] | |||
Dermonecrotic Toxin (DNT) | |||
The secreted neurotoxin, Dermonecrotic Toxin (DNT), causes encephalopathy, which is a fatal complication that effects 1% of patients. DNT targets T-type voltage-gated calcium channel, CaV3, on the central nervous system. Mice injected with DNT showed bleeding of the cerebellum and around the olfactory bulb. [11] The mice injected with DNT showed increased myelin basic protein, a myelination protein, and interleukin-6 protein, a protein produced in response to infections, [12] in the cerebrospinal fluid, indicating inflammation and demyelination in the central nervous system. [11] | |||
Tracheal Cytotoxin: | |||
The tracheal cytotoxin is a cell wall peptidoglycan fragment that kills ciliated respiratory epithelial cells through the production of nitric oxide. It also mediates the production of interleukin 1, a pyrogen. [13] | |||
Adenylate Cyclase Toxin (CyaA): | |||
CyaA is a secreted antigen that is released during early respirator colonization and binds to neutrophils and macrophages. Once it invades the host's immune cells, it is activated by calmodulin and causes a massive increase in cAMP production. CyaA removes two phosphates from ATP to produce cAMP. Because cAMP is a signal for many processes in the cell, its overproduction disrupts many cellular functions. CyaA intoxication leads to apoptosis, weakening the host’s immune response and supporting bacterial colonization. [14] | |||
==References== | ==References== | ||
[1] | [1] Schoch CL, et al. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford). 2020: baaa062. PubMed: 32761142 PMC: PMC7408187 | ||
[2] Mastrantonio, P., Stefanelli, P., Giuliano, M., Herrera Rojas, Y., Ciofi degli Atti, M., Anemona, A., & Tozzi, A. E. (1998). Bordetella parapertussis infection in children: epidemiology, clinical symptoms, and molecular characteristics of isolates. Journal of clinical microbiology, 36(4), 999–1002. https://doi.org/10.1128/JCM.36.4.999-1002.1998 | |||
[3] Parkhill, J., Sebaihia, M., Preston, A. et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet 35, 32–40 (2003). https://doi-org.proxyiub.uits.iu.edu/10.1038/ng1227 | |||
[4] Safarchi, A., Saedi, S., Tay, C. Y., Lamichhane, B., Nakhost Lotfi, M., & Shahcheraghi, F. (2022). Genome Characteristic of Bordetella parapertussis Isolated from Iran. Current microbiology, 79(10), 314. https://doi.org/10.1007/s00284-022-03009-x | |||
[5] Brinig, M. M., Register, K. B., Ackermann, M. R., & Relman, D. A. (2006). Genomic features of Bordetella parapertussis clades with distinct host species specificity. Genome biology, 7(9), R81. https://doi.org/10.1186/gb-2006-7-9-r81 | |||
[6] Ryan, K. J. (2004). Haemophilus and Bordetella . In K. J. Ryan, & C. G. Ray (Eds.), Sherris Medical Microbiology (4th ed., pp. 395-420). USA: The McGraw-Hill Companies, Inc. | |||
[7] Ma, L., Caulfield, A., Dewan, K. K., & Harvill, E. T. (2021). Pertactin-Deficient Bordetella pertussis, Vaccine-Driven Evolution, and Reemergence of Pertussis. Emerging infectious diseases, 27(6), 1561–1566. https://doi.org/10.3201/eid2706.203850 | |||
[8] Iyer, S. S., & Cheng, G. (2012). Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Critical reviews in immunology, 32(1), 23–63. https://doi.org/10.1615/critrevimmunol.v32.i1.30 | |||
[9] Liu, J., Cao, S., Kim, S., Chung, E. Y., Homma, Y., Guan, X., Jimenez, V., & Ma, X. (2005). Interleukin-12: an update on its immunological activities, signaling and regulation of gene expression. Current immunology reviews, 1(2), 119–137. https://doi.org/10.2174/1573395054065115 | |||
[10] Luckheeram, R. V., Zhou, R., Verma, A. D., & Xia, B. (2012). CD4⁺T cells: differentiation and functions. Clinical & developmental immunology, 2012, 925135. https://doi.org/10.1155/2012/925135 | |||
[11] Teruya S, Hiramatsu Y, Nakamura KFukui-Miyazaki ATsukamoto K, Shinoda N, Motooka D, Nakamura S, Ishigaki K, Shinzawa NNishida T, Sugihara F, Maeda Y, Horiguchi Y. 2020. Bordetella Dermonecrotic Toxin Is a Neurotropic Virulence Factor That Uses CaV3.1 as the Cell Surface Receptor. mBio 11:10.1128/mbio.03146-19. | |||
https://doi.org/10.1128/mbio.03146-19 | |||
[12] Tanaka, T., Narazaki, M., & Kishimoto, T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harbor perspectives in biology, 6(10), a016295. https://doi.org/10.1101/cshperspect.a016295 | |||
[13] Heiss, L. N., Moser, S. A., Unanue, E. R., & Goldman, W. E. (1993). Interleukin-1 is linked to the respiratory epithelial cytopathology of pertussis. Infection and immunity, 61(8), 3123–3128. https://doi.org/10.1128/iai.61.8.3123-3128.1993 | |||
[14] Pierre Guermonprez, Nadia Khelef, Eric Blouin, Philippe Rieu, Paola Ricciardi-Castagnoli, Nicole Guiso, Daniel Ladant, Claude Leclerc; The Adenylate Cyclase Toxin of Bordetella pertussis Binds to Target Cells via the αMβ2 Integrin (Cd11b/Cd18). J Exp Med 7 May 2001; 193 (9): 1035–1044. doi: https://doi.org/10.1084/jem.193.9.1035 | |||
[15] Dupré E, Herrou J, Lensink MF, Wintjens R, Vagin A, Lebedev A, et al. (2015) Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS. PLoS Pathog 11(3): e1004700. doi:10.1371/journal.ppat.1004700 | |||
==Author== | ==Author== | ||
Latest revision as of 04:33, 26 April 2024
Classification
Domain: Bacteria
Phylum: Proteobacteria
Class: Betaproteobacteria
Order: Burkholderiales
Family: Alcaligenaceae
Genus and Species: Bordetella parapertussis [1]
Species
Bordetella parapertussis
Description and Significance
Bordetella parapertussis is a Gram-negative, non-motile coccobacillus. It is an obligate aerobe, and inhabits the ciliated upper respiratory epithelial cells of its hosts. Bordetella parapertussis has two hosts, sheep and humans. The two hosts possess two different strains B. parapertussis (ov) infecting sheep and B. parapertussis (hu) infecting humans. These two strains are completely isolated from each other and are genetically distinct, with cross-contamination being extremely rare. Their genetically distinct features indicate independent evolution from a common ancestor, B. bronchiseptica. [3]
Bordetella parapertussis causes a highly infectious upper respiratory tract infection characterized by pertussis, an intense episode of coughing, along with symptoms such as whooping, paroxysm, apnea, vomiting, encephalopathy, and cyanosis. [2] B. parapertussis is most severe in young infants less than six months of age. [4] Bordetella parapertussis usually produces a milder form of whooping cough compared to Bordetella pertussis, the main cause of whooping cough worldwide, however, the epidemiology of the two are not easily distinguished. [2] B. parapertussis is estimated to make up 5-30% of whooping cough cases, particularly in regions with acellular pertussis immunization programs (ACV vaccinations). [4]
The ACV vaccine targets the virulence factors of B. pertussis, which imposes selective pressure on B. parapertussis. B. parapertussis shares some, but not all of the virulence factors with B. pertussis. Therefore, rates of B. parapertussis cases are increasing worldwide because B. parapertussis can evade the ACV vaccine. Additionally, B. parapertussis strains with mutations that eliminate virulence factors, such the increasingly common PRN-negative B. parapertussis infecting humans, pose the greatest risk of becoming endemic due to the fact that the ACV vaccine is unable to use virulence factor mediated phagocytosis. [5] Given its highly infectious spread through respiratory droplets and its ability to evade current vaccines, ongoing research and surveillance efforts to understand B. parapertussis pathogenesis is crucial for disease management and vaccination strategies.
Genome Structure
Bordetella parapertussis possesses a single circular chromosome, with a genome size of approximately 4.7 million base pairs (Mbp) and a G+C content of 65%. [4] Approximately 3,000 genes are shared between B. pertussis, B. bronchiseptica, and B. parapertussis. Both B. parapertussis and B. pertussis evolved from B. bronchiseptica. Only 50 genes are unique to B. parapertussis. [3] B. parapertussis shares a common ancestor with B. pertussis, the main cause of whooping cough worldwide, and has two distinct lineages from which it evolved independently from B. bronchiseptica.
One strain of B. parapertussis causes a milder form of whooping cough in humans, B. parapertussis (hu), and the other infects sheep, B. parapertussis (ov). Both strains are almost entirely isolated from each other and there is little to no genetic exchange. B. parapertussis (hu) possesses 217 pseudogenes, has only one circular chromosome, and is more genetically uniform compared to B. parapertussis (ov). B. parapertussis (ov) possesses 389 pseudogenes, is more genetically diverse, and has an additional plasmid that is 12 kilobases (Kb). [5] Unlike B. pertussis, B. parapertussis does not produce the pertussis toxin that makes B. pertussis so severe. Additionally, B. parapertussis also possesses a pseudogene for flagella, making it nonmotile, unlike B. bronchiseptica. [4]
Cell Structure, Metabolism and Life Cycle
Bordetella parapertussis is a Gram-negative, non-motile, coccobacillus that is about 0.5-1.0 μm in length. [3] It can occur either singularly or in pairs. Therefore, it possesses a thin murein layer and a surrounding outer layer of lipopolysaccharide and phospholipids. It also produces a capsule. It is an obligate aerobe and a chemoorganoheterotroph. The primary source of carbon for Bordetella parapertussis is glutamate. It does not metabolize sugars because it lacks the genes for glucokinase, phosphofructokinase, and fructose-1,6-bisphosphate. It also does not perform fermentation or the TCA cycle. B. parapertussis also does not use cytochrome c oxidase during respiratory metabolism. [3] Additionally, because Bordetella parapertussis requires iron to survive, it performs hemolysis to absorb iron through siderophores. [6]
Bordetella parapertussis is transmitted through respiratory droplets and cannot survive in the outside environment. Upon entering the host, B. parapertussis encounters its optimal growth temperature (37°C), which activates the BvgA/S pathway. The BvgA/S pathway initiates the transcription of virulence-activating genes, which signals host colonization of the ciliated respiratory epithelial cells. B. parapertussis possesses many virulence factors that sustain its growth and reproduction. [7]
Ecology and Pathogenesis
Filamentous Hemagglutinin (FMA): One of the most notable virulence factors is Filamentous Hemagglutinin (FHA). FHA is a long, rod-shaped protein that is both a surface antigen and a secreted protein. As a surface antigen, it binds to ciliated respiratory epithelial cells and allows tracheal colonization. FHA promotes Interleukin 10, a cytokine with anti-inflammatory properties, [8] and reduces Interleukin 12, which promotes both the innate and adaptive immune response. [9] When FHA is secreted it binds to monocytes where it inhibits antigen-dependent CD4+ T cell proliferation and generates apoptosis. [=====16] CD4+ T cells rely on antigen presenting monocytes to activate. CD4+ T cells are responsible for producing cytokines, which help activate the specific immune response, and recruit Macrophages, cytotoxic T cells, and B cells. Suppression of the specific immune response limits destruction of the pathogen by macrophages and cytotoxic T cells and the production of neutralizing antibodies by B cells. [10]
Pertactin (PRN):
Another important virulence factor is Pertactin (PRN). It is an autotransporter responsible for adhesion to the ciliated respiratory cells. PRN is comprised of 16 right-handed parallel β-helixes. It is the largest β-helix structure ever recorded. The ACV vaccine targets PRN for phagocytosis through a cascade of immunological responses mediated by C1q. When antibodies bind to the PRN antigen, they arrange on the bacterial surface in a conformation favorable for C1q binding. C1q binding activates a cascade of synergistic activities: the formation of a Membrane Attack Complex (MAC) which forms pores in the bacterial surface leading to osmolysis, the depositing of C3b factors which act as opsonins (or tags for immune system recognition), and the binding of FcR (fragment crystallizable region) to PRNs to activate phagocytosis. [7] PRN is an important target for the ACV vaccine. However, PRN-negative B. parapertussis is becoming increasing common worldwide, likely due to the selective pressures of the ACV vaccine. PRN-negative B. parapertussis were shown to have either one or both nonsense mutations: either a deletion of an adenine in region I or a guanine deletion in region II of the PRN gene. [4]
Dermonecrotic Toxin (DNT)
The secreted neurotoxin, Dermonecrotic Toxin (DNT), causes encephalopathy, which is a fatal complication that effects 1% of patients. DNT targets T-type voltage-gated calcium channel, CaV3, on the central nervous system. Mice injected with DNT showed bleeding of the cerebellum and around the olfactory bulb. [11] The mice injected with DNT showed increased myelin basic protein, a myelination protein, and interleukin-6 protein, a protein produced in response to infections, [12] in the cerebrospinal fluid, indicating inflammation and demyelination in the central nervous system. [11]
Tracheal Cytotoxin:
The tracheal cytotoxin is a cell wall peptidoglycan fragment that kills ciliated respiratory epithelial cells through the production of nitric oxide. It also mediates the production of interleukin 1, a pyrogen. [13]
Adenylate Cyclase Toxin (CyaA):
CyaA is a secreted antigen that is released during early respirator colonization and binds to neutrophils and macrophages. Once it invades the host's immune cells, it is activated by calmodulin and causes a massive increase in cAMP production. CyaA removes two phosphates from ATP to produce cAMP. Because cAMP is a signal for many processes in the cell, its overproduction disrupts many cellular functions. CyaA intoxication leads to apoptosis, weakening the host’s immune response and supporting bacterial colonization. [14]
References
[1] Schoch CL, et al. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford). 2020: baaa062. PubMed: 32761142 PMC: PMC7408187
[2] Mastrantonio, P., Stefanelli, P., Giuliano, M., Herrera Rojas, Y., Ciofi degli Atti, M., Anemona, A., & Tozzi, A. E. (1998). Bordetella parapertussis infection in children: epidemiology, clinical symptoms, and molecular characteristics of isolates. Journal of clinical microbiology, 36(4), 999–1002. https://doi.org/10.1128/JCM.36.4.999-1002.1998
[3] Parkhill, J., Sebaihia, M., Preston, A. et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet 35, 32–40 (2003). https://doi-org.proxyiub.uits.iu.edu/10.1038/ng1227
[4] Safarchi, A., Saedi, S., Tay, C. Y., Lamichhane, B., Nakhost Lotfi, M., & Shahcheraghi, F. (2022). Genome Characteristic of Bordetella parapertussis Isolated from Iran. Current microbiology, 79(10), 314. https://doi.org/10.1007/s00284-022-03009-x
[5] Brinig, M. M., Register, K. B., Ackermann, M. R., & Relman, D. A. (2006). Genomic features of Bordetella parapertussis clades with distinct host species specificity. Genome biology, 7(9), R81. https://doi.org/10.1186/gb-2006-7-9-r81
[6] Ryan, K. J. (2004). Haemophilus and Bordetella . In K. J. Ryan, & C. G. Ray (Eds.), Sherris Medical Microbiology (4th ed., pp. 395-420). USA: The McGraw-Hill Companies, Inc.
[7] Ma, L., Caulfield, A., Dewan, K. K., & Harvill, E. T. (2021). Pertactin-Deficient Bordetella pertussis, Vaccine-Driven Evolution, and Reemergence of Pertussis. Emerging infectious diseases, 27(6), 1561–1566. https://doi.org/10.3201/eid2706.203850
[8] Iyer, S. S., & Cheng, G. (2012). Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Critical reviews in immunology, 32(1), 23–63. https://doi.org/10.1615/critrevimmunol.v32.i1.30
[9] Liu, J., Cao, S., Kim, S., Chung, E. Y., Homma, Y., Guan, X., Jimenez, V., & Ma, X. (2005). Interleukin-12: an update on its immunological activities, signaling and regulation of gene expression. Current immunology reviews, 1(2), 119–137. https://doi.org/10.2174/1573395054065115
[10] Luckheeram, R. V., Zhou, R., Verma, A. D., & Xia, B. (2012). CD4⁺T cells: differentiation and functions. Clinical & developmental immunology, 2012, 925135. https://doi.org/10.1155/2012/925135
[11] Teruya S, Hiramatsu Y, Nakamura KFukui-Miyazaki ATsukamoto K, Shinoda N, Motooka D, Nakamura S, Ishigaki K, Shinzawa NNishida T, Sugihara F, Maeda Y, Horiguchi Y. 2020. Bordetella Dermonecrotic Toxin Is a Neurotropic Virulence Factor That Uses CaV3.1 as the Cell Surface Receptor. mBio 11:10.1128/mbio.03146-19. https://doi.org/10.1128/mbio.03146-19
[12] Tanaka, T., Narazaki, M., & Kishimoto, T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harbor perspectives in biology, 6(10), a016295. https://doi.org/10.1101/cshperspect.a016295
[13] Heiss, L. N., Moser, S. A., Unanue, E. R., & Goldman, W. E. (1993). Interleukin-1 is linked to the respiratory epithelial cytopathology of pertussis. Infection and immunity, 61(8), 3123–3128. https://doi.org/10.1128/iai.61.8.3123-3128.1993
[14] Pierre Guermonprez, Nadia Khelef, Eric Blouin, Philippe Rieu, Paola Ricciardi-Castagnoli, Nicole Guiso, Daniel Ladant, Claude Leclerc; The Adenylate Cyclase Toxin of Bordetella pertussis Binds to Target Cells via the αMβ2 Integrin (Cd11b/Cd18). J Exp Med 7 May 2001; 193 (9): 1035–1044. doi: https://doi.org/10.1084/jem.193.9.1035
[15] Dupré E, Herrou J, Lensink MF, Wintjens R, Vagin A, Lebedev A, et al. (2015) Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS. PLoS Pathog 11(3): e1004700. doi:10.1371/journal.ppat.1004700
Author
Page authored by Sachin Gupta and Erin Goertzen, students of Prof. Jay Lennon at Indiana University.