Burkholderia mallei: Difference between revisions
| (63 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
==Classification== | ==Classification== | ||
[[File:Screenshot 2024-04-25 at 8.51.02 PM.png|right|280px]] | |||
Domain: Bacteria | Domain: Bacteria | ||
| Line 24: | Line 25: | ||
==Description and Significance== | ==Description and Significance== | ||
[[File: Screenshot 2024-04-25 at 9.44.28 PM.png |left|200px]] | |||
''Burkholderia mallei'' is a Gram-negative, bipolar, aerobic, non-motile bacterium that does not produce spores. It is a small, rod-shaped bacterium that develops slowly in most culture conditions and is a facultative intracellular pathogen. This host-adapted bacterium may survive in the environment for a brief time and is responsible for glanders, a zoonotic illness that mostly affects horse populations and other animals but is rare in humans.[1] ''B. mallei'' is closely related to ''B. pseudomallei'', and genetic studies indicate that it arose as a subspecies of ''B. pseudomallei'' via selective reduction and deletions from the ''B. pseudomallei'' genome, most likely adapting to an intracellular lifestyle within animal hosts.[2] ''B. mallei'', unlike ''B. pseudomallei'' and other members of the genus, is non-motile and has a coccobacillary shape, measuring 1.5-3.0 μm in length, 0.5-1.0 μm in diameter, and rounded ends. | ''Burkholderia mallei'' is a Gram-negative, bipolar, aerobic, non-motile bacterium that does not produce spores. It is a small, rod-shaped bacterium that develops slowly in most culture conditions and is a facultative intracellular pathogen. This host-adapted bacterium may survive in the environment for a brief time and is responsible for glanders, a zoonotic illness that mostly affects horse populations and other animals but is rare in humans.[1] ''B. mallei'' is closely related to ''B. pseudomallei'', and genetic studies indicate that it arose as a subspecies of ''B. pseudomallei'' via selective reduction and deletions from the ''B. pseudomallei'' genome, most likely adapting to an intracellular lifestyle within animal hosts.[2] ''B. mallei'', unlike ''B. pseudomallei'' and other members of the genus, is non-motile and has a coccobacillary shape, measuring 1.5-3.0 μm in length, 0.5-1.0 μm in diameter, and rounded ends. | ||
[[File: Screenshot 2024-04-25 at 10.01.39 PM.png|right|180px]] | |||
The Centers for Disease Control and Prevention (CDC) classifies ''B. mallei'' as a category B select agent due to its high infectiousness, potential for biological warfare and bioterrorism, and hazard to public health and agriculture.[ | The Centers for Disease Control and Prevention (CDC) classifies ''B. mallei'' as a category B select agent due to its high infectiousness, potential for biological warfare and bioterrorism, and hazard to public health and agriculture.[3] The development of improved diagnostic tools, vaccines, and therapeutic strategies for this pathogen is important. ''B. mallei'' was one of the earliest biological warfare agents utilized in the nineteenth century, particularly during World War I, when German agents purposely infected horses and livestock in the United States, Spain, Norway, and other nations via inoculation and feed contamination.[4]While ''B. mallei'' cannot survive outside of its host, thus restricting its utility as a bioweapon, other countries, such as Japan and Russia, have investigated it as a potential biological weapon. Despite its inability to survive outside of a host, ''B. mallei'''s high infectivity and potential for serious harm to human and animal populations make it a troubling virus that necessitates continued research and preparedness measures.[5] | ||
==Genome Structure== | ==Genome Structure== | ||
[[File: Screenshot 2024-04-25 at 10.08.38 PM.png|right|180px]] | |||
B. mallei's genome provides insights into its phylogenetic relationship with other members of the Burkholderias genus and related organisms. Multilocus sequence typing has classified Burkholderia mallei as a subspecies of B. pseudomallei | ''B. mallei's'' genome exhibits distinctive features that separate it from other members of the ''Burkholderia'' genus. Unlike its closely related counterpart, ''B. pseudomallei'' which has a larger genome (around 7.3 Mb) size, B. mallei has a smaller genome with unique characteristics (around 3.5 mb) [1]. While ''B. pseudomallei'' has a larger genome size, ''Burkholderia mallei's'' genome is relatively smaller overall. Which can reflect selective reduction and deletions from the ''B. pseudomallei'' genome during its evolution into a specialized and uniquely distinct pathogen. | ||
B. mallei's genome provides insights into its phylogenetic relationship with other members of the ''Burkholderias'' genus and related organisms. Multilocus sequence typing has classified ''Burkholderia mallei'' as a subspecies of ''B. pseudomallei'' which is suspected to come from clone reduction [4]. This can indicate a close evolutionary relationship [7]. However, genomic analysis reveals that there are distinct genetic differences between the two species, reflecting that their differences in ecological niches and lifestyles as pathogens. Overall the genome is structured as being made up of two circular chromosomes each with their own designated roles. Chromosome one harbors genes for basic cellular functions while chromosome two contains genes associated with adaptation, virulence and pathogenicity[2] . Understanding th genome structure of B. Mallei is crucial for observing its pathogenic mechanisms, evolutionary history and adaptation to its host environment. This is possible from comparative genomic analysis which provides strategies for diagnosis, treatment and prevention of infections caused by the pathogens [2]. | |||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
[[File: Screenshot 2024-04-25 at 10.26.24 PM.png|left|150px]]''B. mallei'' has unique cellular features that contribute majorly to the survival in different host environments. ''B. mallei'' cells are typically coccobacilli shaped, measuring in a range of 1.5–3.0 μm in length and 0.5–1.0 μm in diameter with rounded ends [3] . What separates ''B. mallei'' from its motile counterpart ''B. pseudomallei'' , ''B. mallei'' is nonmotile meaning its not capable of movement on its own. As discussed earlier the cell wall of ''B. mallei'' is Gram-negative, with an outer membrane containing lipopolysaccharides that contribute larg;y to its interaction with host cells. ''B. mallei'' usually produces a thick capsule made up of polysaccharides which are used as a virulence factor protecting the bacteria from host immune defenses [2]. [[File: Screenshot 2024-04-25 at 10.11.53 PM.png |right|200px]] | |||
B. mallei | ''B. mallei'' uses a wide range of metabolic paths to obtain energy and essential nutrients for its growth and survival. ''B. mallei'' is aerobically metabolically active, using aerobic respiration for energy production [7]. The bacteria utilizes organic compounds as carbon and energy sources like sugars, amino acids, and fatty acids, which are catabolized through metabolic pathways and glycolysis. ''B. mallei'' also has nitrogen-fixing capabilities, allowing it to convert nitrogen from the atmosphere into ammonia through nitrogenase enzyme processes [5]. This ability increases its potential to adapt to nitrogen limited environments and contributes to its growth in different ecological environments and conditions. | ||
[[File: Screenshot 2024-04-25 at 10.26.50 PM.png|left|280px]]The life cycle of ''B. mallei'' has many complex interactions with the host's cells and the environment, facilitating its replication and spread. ''B. mallei'' infects host cells [2]. They are able to do this through mechanisms such as adhesion, invasion, and intracellular survival. The bacteria employs virulence factors and secretion systems, to begin infection and evade host defensive responses [4]. Once inside host cells, ''B. mallei'' establishes intracellular replication niches, where it replicates using the hosts cellular machinery and nutrients. The bacterium manipulates host cell signaling pathways and immune responses to create a better suited environment for its survival. ''B. mallei'' eventually triggers host cell lysis, leading to the release of progeny bacteria into the environment. The bacterium can also form biofilm communities in host tissues, which are factors that contribute to the spread and persistence of the infection [6]. | |||
Understanding the cell structure, metabolism, and life cycle of ''B. mallei'' is very important for depicting its pathogenic mechanisms and developing targeted prevention for glanders [1] . More knowledge about the bacterium's metabolic pathways and virulence strategies provide opportunities for the development of potential therapies and vaccines to combat infections caused by this medically important pathogen. | |||
Understanding the cell structure, metabolism, and life cycle of | |||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
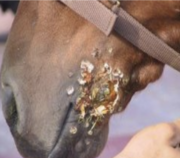
Burkholderia mallei is | ''Burkholderia mallei'' is mainly a pathogen harmful to mammels, with a unqiue effectiveness for equids which are horses, donkeys, and mules [1] . However, it can also infect other species, including humans, dogs, and cats. The bacteria is transmitted through direct contact with infected animals or their secretions, respiratory systems or contaminated bodily fluids. Human infection which ''Burkholderia mallei'' occurs through exposure to infected animals or contaminated environments [5] . Inhalation of the bacteria is the most common mode of transmission, which then leads to the development of glanders. Glanders in humans can manifest as both minor or chronic forms. It's symptoms ranging from fever, chills, and respiratory distress to abscess formation in the lungs, lymph nodes, and skin. In severe cases, spread of the infection may lead to septicemia and multi-organ failure usually killing the infected person. In animals glanders typically occurs through direct contact with infected equids or exposure to contaminated food or water [6] . The bacterium can enter the body through mucous membranes, skin abrasions, or inhalation [4] . Clinical signs of glanders in animals are similar to human effects including fever, nasal discharge, coughing, and ulcerative lesions in the respiratory tract and skin makes the animals look very clearly ill and deathly. Chronic infection can lead to multiple organ failure, and death if left untreated.[[File: Screenshot 2024-04-25 at 10.35.08 PM.png|right|280px]] | ||
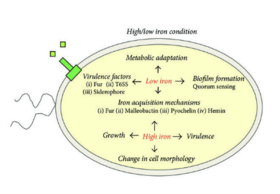
B. mallei has various virulence factors that contribute to its pathogenicity and ability to cause disease in | ''B. mallei'' has various virulence factors that contribute to its pathogenicity and ability to cause disease in hosts [7]. The polysaccharide capsule of ''B. mallei'' serves asthe key virulence factor, providing protection against host immune defenses and facilitating evasion of phagocytosis. The T6SS of ''B. mallei'' is involved in host cell invasion and intracellular survival, allowing the bacteria to manipulate the host cellular processes and establish persistent continuous infections. The LPS molecules on the outer membrane of ''B. mallei'' contribute to its pathogenicity by intiating inflammatory responses and modulating host immune signaling pathways [2]. | ||
B. mallei can persist in the environment for extended periods, particularly in soil and water contaminated with infected animal | ''B. mallei'' can persist in the environment for extended periods of time, particularly in soil and water contaminated with infected animal stool or carcasses [1] . This environment serves as a potential source of infection for hosts. Changes in environmental conditions, such as increased temperatures and precipitation patterns associated with climate change, may influence the distribution and prevalence of ''B. mallei'' and other infectious agents. The impact of climate change on glanders epidemiology could initiate possible investigation and research. | ||
Overall Understanding the ecology and pathogenesis of B. mallei is essential for implementing effective control measures and | Overall Understanding the ecology and pathogenesis of ''B. mallei'' is essential for implementing effective control measures and stopping the spread of glanders in both human and animal populations. Strategies for disease prevention and surveillance like including vaccination, biosecurity measures, and early detection of infected individuals and animals, are crucial for minimizing the impacts of glanders. Although glanders isn't nearly as prevalent as it once was, understanding how the bacteria functions ecologically could support in research and prevention of other diseases. | ||
==References== | ==References== | ||
| Line 61: | Line 61: | ||
4.] Lehavi, O., Aizenstien, O., Katz, L. H., & Hourvitz, A. (2002). ''Harefuah'', 141 Spec No, 88–119 | 4.] Lehavi, O., Aizenstien, O., Katz, L. H., & Hourvitz, A. (2002). ''Harefuah'', 141 Spec No, 88–119 | ||
5.] Wheelis, M. (n.d.). First shots fired in biological warfare. ''Nature News''. https://www.nature.com/articles/26089 | |||
6.] Galyov, E. E., Brett, P. J., & DeShazer, D. (2010, October 13). Molecular insights into burkholderia pseudomallei and burkholderia mallei pathogenesis. Annual Review of Microbiology. https://www.annualreviews.org/content/journals/10.1146/annurev.micro.112408.134030 | |||
7.] Nierman, W. (2004, September 17). PNAS. Structural flexibility in the Burkholderia mallei genome. https://www.pnas.org/ | |||
==Author== | ==Author== | ||
Page authored by Janvi | Page authored by Janvi Desai & Jeremiah Garnica, student of Prof. Jay Lennon at IndianaUniversity. | ||
<!-- Do not remove this line-->[[Category:Pages edited by students of Jay Lennon at Indiana University]] | <!-- Do not remove this line-->[[Category:Pages edited by students of Jay Lennon at Indiana University]] | ||
Latest revision as of 03:48, 26 April 2024
Classification
Domain: Bacteria
Phylum: Pseudomonadota
Class: Betaproteobacteria
Order: Burkholderiales
family: Burkholderiaceae
genus: Burkholderia
Species
Burkholderia mallei
|
NCBI: Taxonomy |
Genus species
Description and Significance
Burkholderia mallei is a Gram-negative, bipolar, aerobic, non-motile bacterium that does not produce spores. It is a small, rod-shaped bacterium that develops slowly in most culture conditions and is a facultative intracellular pathogen. This host-adapted bacterium may survive in the environment for a brief time and is responsible for glanders, a zoonotic illness that mostly affects horse populations and other animals but is rare in humans.[1] B. mallei is closely related to B. pseudomallei, and genetic studies indicate that it arose as a subspecies of B. pseudomallei via selective reduction and deletions from the B. pseudomallei genome, most likely adapting to an intracellular lifestyle within animal hosts.[2] B. mallei, unlike B. pseudomallei and other members of the genus, is non-motile and has a coccobacillary shape, measuring 1.5-3.0 μm in length, 0.5-1.0 μm in diameter, and rounded ends.
The Centers for Disease Control and Prevention (CDC) classifies B. mallei as a category B select agent due to its high infectiousness, potential for biological warfare and bioterrorism, and hazard to public health and agriculture.[3] The development of improved diagnostic tools, vaccines, and therapeutic strategies for this pathogen is important. B. mallei was one of the earliest biological warfare agents utilized in the nineteenth century, particularly during World War I, when German agents purposely infected horses and livestock in the United States, Spain, Norway, and other nations via inoculation and feed contamination.[4]While B. mallei cannot survive outside of its host, thus restricting its utility as a bioweapon, other countries, such as Japan and Russia, have investigated it as a potential biological weapon. Despite its inability to survive outside of a host, B. mallei's high infectivity and potential for serious harm to human and animal populations make it a troubling virus that necessitates continued research and preparedness measures.[5]
Genome Structure
B. mallei's genome exhibits distinctive features that separate it from other members of the Burkholderia genus. Unlike its closely related counterpart, B. pseudomallei which has a larger genome (around 7.3 Mb) size, B. mallei has a smaller genome with unique characteristics (around 3.5 mb) [1]. While B. pseudomallei has a larger genome size, Burkholderia mallei's genome is relatively smaller overall. Which can reflect selective reduction and deletions from the B. pseudomallei genome during its evolution into a specialized and uniquely distinct pathogen. B. mallei's genome provides insights into its phylogenetic relationship with other members of the Burkholderias genus and related organisms. Multilocus sequence typing has classified Burkholderia mallei as a subspecies of B. pseudomallei which is suspected to come from clone reduction [4]. This can indicate a close evolutionary relationship [7]. However, genomic analysis reveals that there are distinct genetic differences between the two species, reflecting that their differences in ecological niches and lifestyles as pathogens. Overall the genome is structured as being made up of two circular chromosomes each with their own designated roles. Chromosome one harbors genes for basic cellular functions while chromosome two contains genes associated with adaptation, virulence and pathogenicity[2] . Understanding th genome structure of B. Mallei is crucial for observing its pathogenic mechanisms, evolutionary history and adaptation to its host environment. This is possible from comparative genomic analysis which provides strategies for diagnosis, treatment and prevention of infections caused by the pathogens [2].
Cell Structure, Metabolism and Life Cycle
B. mallei has unique cellular features that contribute majorly to the survival in different host environments. B. mallei cells are typically coccobacilli shaped, measuring in a range of 1.5–3.0 μm in length and 0.5–1.0 μm in diameter with rounded ends [3] . What separates B. mallei from its motile counterpart B. pseudomallei , B. mallei is nonmotile meaning its not capable of movement on its own. As discussed earlier the cell wall of B. mallei is Gram-negative, with an outer membrane containing lipopolysaccharides that contribute larg;y to its interaction with host cells. B. mallei usually produces a thick capsule made up of polysaccharides which are used as a virulence factor protecting the bacteria from host immune defenses [2].
B. mallei uses a wide range of metabolic paths to obtain energy and essential nutrients for its growth and survival. B. mallei is aerobically metabolically active, using aerobic respiration for energy production [7]. The bacteria utilizes organic compounds as carbon and energy sources like sugars, amino acids, and fatty acids, which are catabolized through metabolic pathways and glycolysis. B. mallei also has nitrogen-fixing capabilities, allowing it to convert nitrogen from the atmosphere into ammonia through nitrogenase enzyme processes [5]. This ability increases its potential to adapt to nitrogen limited environments and contributes to its growth in different ecological environments and conditions.
The life cycle of B. mallei has many complex interactions with the host's cells and the environment, facilitating its replication and spread. B. mallei infects host cells [2]. They are able to do this through mechanisms such as adhesion, invasion, and intracellular survival. The bacteria employs virulence factors and secretion systems, to begin infection and evade host defensive responses [4]. Once inside host cells, B. mallei establishes intracellular replication niches, where it replicates using the hosts cellular machinery and nutrients. The bacterium manipulates host cell signaling pathways and immune responses to create a better suited environment for its survival. B. mallei eventually triggers host cell lysis, leading to the release of progeny bacteria into the environment. The bacterium can also form biofilm communities in host tissues, which are factors that contribute to the spread and persistence of the infection [6].
Understanding the cell structure, metabolism, and life cycle of B. mallei is very important for depicting its pathogenic mechanisms and developing targeted prevention for glanders [1] . More knowledge about the bacterium's metabolic pathways and virulence strategies provide opportunities for the development of potential therapies and vaccines to combat infections caused by this medically important pathogen.
Ecology and Pathogenesis
Burkholderia mallei is mainly a pathogen harmful to mammels, with a unqiue effectiveness for equids which are horses, donkeys, and mules [1] . However, it can also infect other species, including humans, dogs, and cats. The bacteria is transmitted through direct contact with infected animals or their secretions, respiratory systems or contaminated bodily fluids. Human infection which Burkholderia mallei occurs through exposure to infected animals or contaminated environments [5] . Inhalation of the bacteria is the most common mode of transmission, which then leads to the development of glanders. Glanders in humans can manifest as both minor or chronic forms. It's symptoms ranging from fever, chills, and respiratory distress to abscess formation in the lungs, lymph nodes, and skin. In severe cases, spread of the infection may lead to septicemia and multi-organ failure usually killing the infected person. In animals glanders typically occurs through direct contact with infected equids or exposure to contaminated food or water [6] . The bacterium can enter the body through mucous membranes, skin abrasions, or inhalation [4] . Clinical signs of glanders in animals are similar to human effects including fever, nasal discharge, coughing, and ulcerative lesions in the respiratory tract and skin makes the animals look very clearly ill and deathly. Chronic infection can lead to multiple organ failure, and death if left untreated.
B. mallei has various virulence factors that contribute to its pathogenicity and ability to cause disease in hosts [7]. The polysaccharide capsule of B. mallei serves asthe key virulence factor, providing protection against host immune defenses and facilitating evasion of phagocytosis. The T6SS of B. mallei is involved in host cell invasion and intracellular survival, allowing the bacteria to manipulate the host cellular processes and establish persistent continuous infections. The LPS molecules on the outer membrane of B. mallei contribute to its pathogenicity by intiating inflammatory responses and modulating host immune signaling pathways [2].
B. mallei can persist in the environment for extended periods of time, particularly in soil and water contaminated with infected animal stool or carcasses [1] . This environment serves as a potential source of infection for hosts. Changes in environmental conditions, such as increased temperatures and precipitation patterns associated with climate change, may influence the distribution and prevalence of B. mallei and other infectious agents. The impact of climate change on glanders epidemiology could initiate possible investigation and research.
Overall Understanding the ecology and pathogenesis of B. mallei is essential for implementing effective control measures and stopping the spread of glanders in both human and animal populations. Strategies for disease prevention and surveillance like including vaccination, biosecurity measures, and early detection of infected individuals and animals, are crucial for minimizing the impacts of glanders. Although glanders isn't nearly as prevalent as it once was, understanding how the bacteria functions ecologically could support in research and prevention of other diseases.
References
1.] Godoy, D., Randle, G., Simpson, A. J., Aanensen, D. M., Pitt, T. L., Kinoshita, R., & Spratt, B. G. (2003). Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. Journal of clinical microbiology, 41(5), 2068–2079. https://doi.org/10.1128/JCM.41.5.2068-2079.2003
2.] Song, H., Hwang, J., Yi, H., Ulrich, R. L., Yu, Y., Nierman, W. C., & Kim, H. S. (2010). The early stage of bacterial genome-reductive evolution in the host. PLoS pathogens, 6(5), e1000922. https://doi.org/10.1371/journal.ppat.1000922
3.] Centers for Disease Control and Prevention. (2017, October 31). Glanders. Centers for Disease Control and Prevention. https://www.cdc.gov/glanders/
4.] Lehavi, O., Aizenstien, O., Katz, L. H., & Hourvitz, A. (2002). Harefuah, 141 Spec No, 88–119
5.] Wheelis, M. (n.d.). First shots fired in biological warfare. Nature News. https://www.nature.com/articles/26089
6.] Galyov, E. E., Brett, P. J., & DeShazer, D. (2010, October 13). Molecular insights into burkholderia pseudomallei and burkholderia mallei pathogenesis. Annual Review of Microbiology. https://www.annualreviews.org/content/journals/10.1146/annurev.micro.112408.134030
7.] Nierman, W. (2004, September 17). PNAS. Structural flexibility in the Burkholderia mallei genome. https://www.pnas.org/
Author
Page authored by Janvi Desai & Jeremiah Garnica, student of Prof. Jay Lennon at IndianaUniversity.