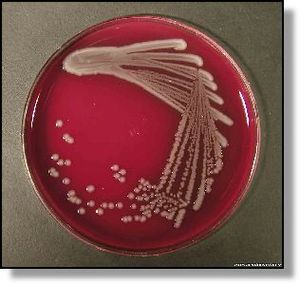
Escherichia coli O157:H7 and Hemolytic uremic syndrome

By [Kye Duren]
Introduction
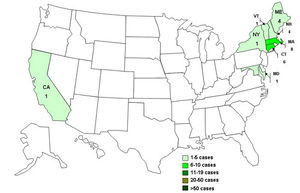
In 2009, the CDC released a report about a multistate outbreak of E.coli in nestle toll house raw cookie dough products. 72 people in 30 states were infected, most people developed diarrhea and cramps, but some developed more severe illnesses like hemolytic uremic syndrome .The microbial culprit for these unfortunate events was E.coli strain O157:H7, a rather hostile foe of our bodies.
Hemolytic uremic syndrome
Hemolytic uremic syndrome (HUS) is a condition resulting from the abnormal premature destruction of red blood cells, usually induced by toxins released from an infection. Damaged red blood cells can clog the filtering system in the kidneys ultimately resulting in life threatening kidney failure. HUS is predominantly more present in children than in adults and this disease is the leading cause of acute renal failure in children, however most children who develop the syndrome can recover without permanent damage to their health. Approximately 15% of those infected with HUS die, or never recover from chronic renal failure. In 1955 Gasser, Gautien, Steath and Siebmann discovered HUS and characterized it as a syndrome. The first outbreak of (HUS) was reported in 1966, when 10 children contracted the disease ranging from ages (6wks-3yrs old). All cases were concentrated within a region of Northern Wales surrounding the city Rhyl (Mclean et al. 1966). Mclean, although he did not know the agent of infection, concluded that because of the epidemic nature of the cases (small geographical are and a small time period), the agent was either toxic or infectious (Mclean et al. 1966). After about twenty years of research, Mclean’s hypothesis was proven correct when (Konowalchuk et al. 1977) determined verotoxin as the chemical cause for (HUS) and within the next five years it E. coli O157; H7 was identified as the infective agent that administered the toxin

.
Clinicalpathology and symptoms of Hemolytic uremic syndrome
E.coliO157:H7 infection can be asymptomatic or produce findings like watery diarrhea, bloody diarrhea, HUS, thrombotic thrombocytopenia pupura, and death. After initial infection E.coliO157:H7 incubates for 3-4 days, patients then begin to develop watery diarrhea. 25-75% recover with no problems, however if the disease progresses, bloody diarrhea begins on day 2 or 3 ranging from streaks of blood to stool that is all blood( Besser et al. 1999). After a week the illness usually dissipates, but 5-10% of children develop Hemolytic uremic syndrome consisting of microangiopathic hemolytic anemia (low red blood cell count), thrombocytopenia (low blood platelet count), and oliguric renal failure( Besser et al. 1999). The incidence of HUS is higher in children and the elderly, although anyone of any age can’t contract the disease. More susceptibility among children and the elderly may be a consequence of an immature, developing immune system in children and a degrading immune system among the elderly. However there is also evidence for age related differences in receptor expression (Besser et al, 2012)
Escherichia .coliO157:H7
This organism is a enterhemorrhagic , rod shaped, gram negative bacterium , with an outer membrane a periplasmic space with a peptidoglycan layer, and an inner, cytoplasmic membrane. Escherichia coli O157:H7 uses oxidative fermentation to metabolize a variety of substrates, except sorbitol, which is a standard that separates this bacterium from non-pathogenic E.coli.
Genome
E.coliO157:H7 has a single circular genome with about 5,498,457 bp. There is also the addition of a large virulence plasmid consisting of 97,721 bp, along with a cryptic plasmid of 3306 bp which equals a total of 5,594,477bp (Hayashi et al. 2001). E.coliO157:H7 contains 1.34 million bp of EHEC specific DNA; 1,387new genes, some of which are related to virulence, while in others the function remains unknown (Donnenberg and Whittam 2001). Virulence factors in any E.coli pathotype come from a variety of places including plasmids, phages, and foreign genomic material. Half (48.2%) of the E.coliO157:H7 specific DNA sequences are of bacteriophage origin (Hayashi et al. 2001). E.coliO157:H7 also contains genes for the biosynthesis of fimbriae and adhesion like proteins which allow these organisms to use a diverse array of adhesion mechanisms , but it is unknown under what circumstances these genes are expressed. The presence of these genes suggests that this bacterium may bind to a variety of surfaces like: tissues, cells, and molecules that were previously unrecognized (Hayashi et al. 2001). E.coliO157:H7 produces shiga-like toxin (STX) which is the active toxin known to cause HUS and its life threatening complications. The genes for STX are encoded on the genome of two lambda –like phages which have lysogenized E.coli pathotypes.
Evolution of pathogenesis in E.coliO157:H7
Evolved from Enteropathogenic E.coli(EPEC) that was lysogenized by a bacteriophage, allowing the bacterium to develop virulence. According to Donnenberg and Whittam, the common ancestor between Enteropathogenic E.coli(EPEC) O55:H7 clone and Enterohemmorrhagic E.coli(EHEC) O157:H7 received its locus for enterocyte effacement (LEE), which provides proteins for the bacteria to adhere to the host cell, from an early EPEC-like ancestor. Next, this EPEC- like bacterium acquired stx2, a gene coding for a type of shiga like-toxin that is the active agent in Hemolytic uremic syndrome. Donnenberg and Whittam believe this occurred via transduction by a toxin-converting bacteriophage, creating virulent E.coli strains. The next step in their evolution involved two changes; EHEC acquired a plasmid and switched from O55 to O157 creating two distinct lines of E.coli. The E.coliO157:H7 two distinct characteristics of commensal strains of E.coli, and acquired the stx1 gene creating the common O157:H7 strain (Donnenberg and Whittam, 2001).
Habitat
Like nonpathogenic strains of E.coli, the O157:H7 serotype lives within the gastrointestinal tract of a host organism. Cattle are the main reservoir for these microbes due to their ability to tolerate them, yet these microbes utilize the same tools to colonize the human colon(Nguyen and Sperandio, 2012). E.coliO157:H7 does not cause any symptoms of HUS, or hemorrhagic colitis, in bovine hosts. Humans express Gb3 on their vascular endothelium which promotes the pathogenesis of shiga- like toxin or verocytotoxin, whereas Cattle lack the expression of vascular endothelium. However cattle express Gb3 in their kidneys and their brains, yet E.coliO157:H7 is unable to permeate blood vessels in the gastrointestinal tract which prevent bacteria from being transmitted to other organs causing vascular damage (Nguyen and Sperandio, 2012).E.coliO157:H7 in human hosts lives within the human colon which cause electrolyte imbalance, while in cattle they colonize the recto-anal junction (RAJ) which allows cattle to be impervious to this microbe’s verocytotoxin.
Acid Resistance
Although ruminant organisms work as excellent hosts for E.coliO157:H7, the microbe must breach an acidic environment in order to reach the preferred (RAJ) region. E.coli O157:H7 enters the gastrointestinal tract of cattle through the rumen where it must breach four stomachs to reach the (RAJ). E.coliO157:H7 have developed three acid resistance systems to be able to survive these conditions. Acid resistance (AR) System 1 involves the repression of glucose or oxidation, yet the mechanism of this system isn’t well known. (AR )system 2 is glutamate-dependent and (AR )system 3 Arginine-dependent, but the mode of action is similar between these species. These systems use the Arginine decarboxylase AdiA and glutamate decarboxylases GadA and GadB to convert arginine and glutamate into agmatine and GABA respectively. In each case the α carboxyl group on each amino acid is displaced by a proton from the cytoplasm. This uptake of protons increases internal pH of the cell allowing E.coliO157:H7 maintain homeostasis in highly acidic cattle stomachs (Nguyen and Sperandio 2012).
Mechanism of Adhesion

]The most well understood method of Adhesion for E.coliO157:H7 and in other enterohemorrhagic bacteria is the formation of an A/E lesion (Nguyen and Sperandio, 2012). E.coliO157:H7 forms attaching A/E lesions at the RAJ allowing this bacterium to gain foothold in the gastrointestinal tract. The genes required for this process are located on the locus for enterocyte effacement (LEE). This locus consists of 41 genes is placed into five operons including LEE5, which encodes for the type 3 secretion system (T3SS) encompassing regulator proteins, chaperones, and effector proteins, and LEE1 , the master transcription factor of the pathogenic island LEE is derived from. The T3SS complex resembles a molecular syringe where E.coliO157:H7 injects its effector proteins including (Tir) into the cytoplasm of the target cell (Nguyen and Sperandio, 2012) . Tir is a protein that directs itself to the cytoplasmic membrane and injects itself leaving its central domain exposed (Nguyen and Sperandio, 2012). Tir’s central domain interacts with intimin, an important LEE effector protein, to from a tight attachment to the target eukaryotic cell (Nguyen and Sperandio, 2012). EspFu is a non-LEE protein that is secreted into the target cell in order to recruit host cytoskeleton and actin polymerizing species to subvert the target cell (Nguyen and Sperandio, 2012). The protein elicits the actin nucleotide promoting factor Wriskott Aldrich syndrome protein (N-WASP) and the insulin receptor tyrosine kinase (Nguyen and Sperandio, 2012). Actin then accumulates within the cell underneath the bacteria causing a lesion among the cytoplasm. LEE mediated adherence to intestinal epithelia is essential in E.coliO157:H7's ability to colonize both ruminant and human gastrointestinal tracts.
Alternative mechanisms for Adhesion
E.coliO157:H7demonstrate other methods to maintain attached to their preferred habitats. Deferred Adherence describes an evenly distributed surface of bacteria over the surface of epithelial cells. Localized adherence describes the formation of tight clusters of micro colonies at multiple specific sites on an epithelial cell surface. Finally there was evidence of a human ileocecal cell “log jam,” that described bacterial adherence at junctions between each cell. This form of adherence is also seen among other related strains of E.coli.
Transmission
1% of all healthy cattle have E.coliO157:H7 within their gastrointestinal tract (Besser et al. 2001). Cattle transmit E.coli to humans by shedding them in their feces. A proportion of cattle are called super shedders because they excrete more E.coli O157:H7 than others. They make up a very small ratio among cattle however they produce 95% of all theE.coliO157:H7 shed. High concentration of E.coliO157:H7 in feces result from intimate colonization at the (RAJ). Once the bacterium is shed humans acquire E.coli by consuming contaminated bovine derived products: meat, milk, dairy products, contaminated water, unpasteurized apple drinks, and vegetables (Nguyen and Sperandio, 2012). E.coliO157:H7 contamination of lettuce and spinach seedlings were shown to be higher in a growth chamber than in environmental conditions (Erickson et al. 2014). The lack of bacterial internalization of the microbe into the produce, suggests that the growth and development of E.coliO157:H7 is susceptible to competition with native soil bacteria and other environmental elements that cause stress to this pathogen(Erickson et al. 2014). The bacterium may also spread from person to person surrounding daycare centers and chronic care facilities (Besser et al. 2001). E.coli O157:H7 has a small infectious dose of 10-100 organisms, in fact less than 50 organisms has been shown to cause infection (Nguyen and Sperandio, 2012).
Pathogenesis
E.coliO157:H7 utilize the same mechanisms used to colonize (RAJ) in order to colonize the human colon including Acid resistance and (A/E) lesion adherence. In the human body when E.coliO157:H7 cells are ingested, the must travel through our stomach and use there acid resistance systems to survive that environment on their way to the colon where the bacteria adhere to epithelial cell surfaces in the colon.
Shiga like Toxin( Verocytotoxin)
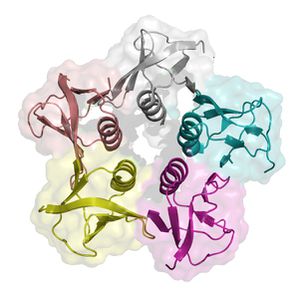
Shiga-like toxins (STX) get their name from from being extremely similar to shiga-toxin that live within Shigella dysenteriea. This substance is a compound toxin comprised of two parts. The A subunit is a single catalytic unit made up of amino acid residue , while the B subunit is a multimeric unit consisting of five monomers that has the purpose of binding a specific glycolipid receptor. The preferential translation of the B subunit is the result of a stronger ribosomal binding site on the B subunit gene (Williams et al. 1999). The residues on the A subunit unify to form an alpha helix which penetrates a pore within the B subunit pentamer. The specific eukaryotic cell surface receptor for (STX) proteins is the globotriaosylceramide (Gb3) protein receptor. STX has two possible binding sites on either side of the residue. One site is between adjacent B monomers and another is in an indentation on the B subunit surface opposed to the plasma membrane. There is also a third binding site located on Trp 34, however this site is putative (Paton and Paton, 1998). Once shiga-like toxin is bound to the target cell, the toxin itself is thought to be internalized by a endocytosis mediated by the epithelial cell receptors (Paton and Paton, 1998).
Roles of A and B subunits in Pathogenesis
The B subunit binds to a terminal di galactose on the cell membrane that exhibits Gb3 receptors. Once the toxin is internalized, the A subunit is cleaved ubiquitously by furin in the host eukaryotic cell. The cleaved A subunit moves into the cytosol, (mechanism unknown), and cleaves an adenine from the 28S rRNA where amino acyl transfer RNA bind, actively truncating protein synthesis (Williams et al. 1999). Protein synthesis inhibition of epithelial cells caused apoptosis in cells that had internalized STX. Protein synthesis inhibition was shown to have large effects on glomerular and cortical tubular epithelial cells (Williams et al. 1999). Epithelial cells were shown to be some of the most sensitive cells to intoxication by shiga-like toxin (Williams et al. 1999). (STX) enter the bloodstream after translocation across epithelial cells lining the intestines and damage endothelial cells which lead to coagulation cascades, formation of microthrombli, intravascular hemolysis, and ischemia. Major portions of histopathological lesions are associated with HC and HUS and Capillary occlusion results in reduced blood flow to kidneys leading to possible renal failure. Endothelial cells appear to be particularly sensitive to shiga-like toxin. Sodium butyrate can also sensitize endothelial cells by causing the up regulation of Gb3 receptors. It’s hypothesized that during an E.coli O157:H7 infection, damage within the gastrointestinal tract may result in increased exposure of endothelial cells to butyrate, which is in abundance within the colon, contributing to pathogenesis.
Global Impact of Escherichia coli O157:H7
E.coliO157:H7 has become a common bacterial cause of diarrhea in the US.E.coliO157:H7 is the leading cause kidney failure in children. There is an estimated 20,000 cases of E.coliO157:H7 infection every year in the US, it is also the leading cause hemolytic uremic syndrome in the US, Canada, and Europe(Besser et al. 1999). This bacterium can also have an impact on a country’s economy. In Canada alone it’s estimated that 60,211 patients are infected by E.coliO157:H7 annually, which costs the country a total of 403.9 million dollars a year. Annually, 22,344 of the cases are first time infections that cost 26.7 million dollars, while 37,867 of the cases belong to long-term health outcomes which cost 377.2 million each year (Sockett et al. 2014).
Prevention & Treatment
Prevention can be seen as a viable method of treatment for E.coliO157:H7 infection in humans. According to the Center for Disease Control (CDC), good hygiene and hand washing especially after using the bathroom, changing a diaper, cooking or eating food, touching or petting animals, can help prevent transmission. Some other interesting methods of prevention include knowing your susceptibility to food poisoning by age or health condition, cooking meat thoroughly, maintaining a clean kitchen, avoiding the consumption of raw or unpasteurized food items like milk or cookie dough, and avoiding water intake while swimming.
Currently there is no foundational treatment available toxin Hemolytic uremic syndrome (Nguyen and Sperandio, 2012) . Antibiotics exacerbate shiga-toxin mediated toxicity. Patients treated with antibiotics were at higher risk for developing HUS. Pre-emptive antibiotics, administered during then Hemorrhagic colitis stage were also shown to increase chances of developing HUS (Nguyen and Sperandio, 2012). Antibiotics promote STX production by increasing replication and expression of STX genes. The STX induction promotes phage mediated lysis of E.coliO157:H7, and since STX lies within a lambda-like prophage, this may induce lyctic cycling within the bacteria causing further release of Shiga-toxin into the gastrointestinal tract. When trying to treat a patient with HUS one may use intensive support to maintain homeostasis including: peritoneal dialysis, hemodialysis, fluid balance, and the treatment of hypertension. Synorbs Pk is a new drug designed to bind all shiga-like toxin and neutralize their effects. Synorbs Pk consists of Gb3 receptors that will attract and bind with remaining extracellular STX that hasn’t permeated epithelial cells. However this form of treatment does not affect STX already internalized by epithelial cells (Paton and Paton, 1998).
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2013, Kenyon College.