Livestock Pathogens and Human Consequences: Case Studies of RVF and Maedi Visna: Difference between revisions
No edit summary |
No edit summary |
||
| Line 23: | Line 23: | ||
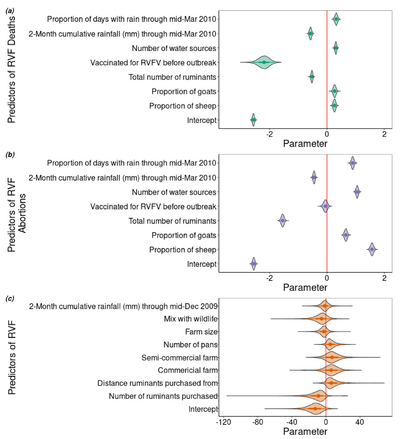
[[Image: Predictors of RVF.png |thumb|400px|left| Figure 2:Factors that predict RVF deaths, abortions, and infection during 2010 Rift Valley Outbreak in Central South Africa. Photo Credit: <ref>[https://www.mdpi.com/2076-0817/9/11/914 Rostal, M. et al.: Farm-Level Risk Factors of Increased Abortion and Mortality in Domestic Ruminants during the 2010 Rift Valley Fever Outbreak in Central South Africa. Pathogens 2020 9:94.]</ref> https://www.mdpi.com/2076-0817/9/11/914]] | [[Image: Predictors of RVF.png |thumb|400px|left| Figure 2:Factors that predict RVF deaths, abortions, and infection during 2010 Rift Valley Outbreak in Central South Africa. Photo Credit: <ref>[https://www.mdpi.com/2076-0817/9/11/914 Rostal, M. et al.: Farm-Level Risk Factors of Increased Abortion and Mortality in Domestic Ruminants during the 2010 Rift Valley Fever Outbreak in Central South Africa. Pathogens 2020 9:94.]</ref> https://www.mdpi.com/2076-0817/9/11/914]] | ||
<br><b>Diagnosis</b> | <br><b>Diagnosis</b> | ||
<br>Because the disease is nonspecific, conditional factors can often give an indication that there is an outbreak of RVF. Factors like heavy rainfall, and large mosquito populations act as predictors for outbreaks (figure 4) and significant abortions post infection can be a sign that there is a current outbreak <ref name=Chengula/> <ref name= MerckRift/>. Tissues from recently aborted fetuses from cattle or sheep contain high titers of the virus and can be used for its isolation. Serum or whole blood samples from animals in the febrile stage of the disease can also be readily used for viral isolation. However, the procedures for viral isolation are slow and expensive so new technologies were vital to accommodate the need for rapid assessment of RVF infection <ref name= Pepin1/>. This came in the form of real-time reverse-transcription loop mediated isothermal amplification assays (RT-LAMP). These RT-LAMP assays had the benefits of high diagnostic accuracy within 30 minutes and are manufactured with relatively inexpensive materials, making it a promising diagnostic tool not only for global health NGOs but also for the people who raise livestock <ref name= Pepin1/. <br> | <br>Because the disease is nonspecific, conditional factors can often give an indication that there is an outbreak of RVF. Factors like heavy rainfall, and large mosquito populations act as predictors for outbreaks (figure 4) and significant abortions post infection can be a sign that there is a current outbreak <ref name=Chengula/> <ref name= MerckRift/>. Tissues from recently aborted fetuses from cattle or sheep contain high titers of the virus and can be used for its isolation. Serum or whole blood samples from animals in the febrile stage of the disease can also be readily used for viral isolation. However, the procedures for viral isolation are slow and expensive so new technologies were vital to accommodate the need for rapid assessment of RVF infection <ref name= Pepin1/>. This came in the form of real-time reverse-transcription loop mediated isothermal amplification assays (RT-LAMP). These RT-LAMP assays had the benefits of high diagnostic accuracy within 30 minutes and are manufactured with relatively inexpensive materials, making it a promising diagnostic tool not only for global health NGOs but also for the people who raise livestock <ref name= Pepin1/>. <br> | ||
<br><b>Treament and Prevention</b> | <br><b>Treament and Prevention</b> | ||
<br>There is no current treatment for animals with RVF leaving vaccination as the only broadly applicable prevention strategy. There have been several vaccines developed which have seen varying levels of success. The earliest vaccine for RVF was developed in 1949 and is still used today, although its use of a modified live vaccine (Smithburn) retains some virulence and is known to induce abortions and does not produce sufficient antibody response in cattle <ref name= Pepin1/> <ref name= MerckRift/>. More recent attempts at a vaccine have proved promising with a live-attenuated vaccine recently being approved in South Africa and some recombinant vaccines expressing the RVFV surface glycoproteins are in development <ref name= Pepin1/> <ref name= CDCrift/> <ref name= MerckRift/>. However, in most places, RVF does not have long lasting endemic infection and so vaccines are used as an emergency measure in the event of an outbreak <ref name= MerckRift/>. | <br>There is no current treatment for animals with RVF leaving vaccination as the only broadly applicable prevention strategy. There have been several vaccines developed which have seen varying levels of success. The earliest vaccine for RVF was developed in 1949 and is still used today, although its use of a modified live vaccine (Smithburn) retains some virulence and is known to induce abortions and does not produce sufficient antibody response in cattle <ref name= Pepin1/> <ref name= MerckRift/>. More recent attempts at a vaccine have proved promising with a live-attenuated vaccine recently being approved in South Africa and some recombinant vaccines expressing the RVFV surface glycoproteins are in development <ref name= Pepin1/> <ref name= CDCrift/> <ref name= MerckRift/>. However, in most places, RVF does not have long lasting endemic infection and so vaccines are used as an emergency measure in the event of an outbreak <ref name= MerckRift/>. | ||
Revision as of 17:36, 17 April 2024
Introduction
By Jack Caine
For thousands of years humans have cultivated animals as companions and food sources. This long-lasting and close relationship, in addition to providing a foundation for human global expansion, has exposed us to many of their pathogens [1]. While these diseases can be quite deadly in their original hosts, the opportunity for a pathogen to be transmitted to a human from an animal increases with duration with them. Zoonoses are any disease that originates in animals and is transmitted to humans [2]. Nearly 60% of known infectious diseases come from zoonotic sources [1]. While zoonoses are not the most common type of disease in humans they do have the capacity to have outbreaks which can result in epidemics or pandemics. Many of the world’s most recent and devastating epidemics and pandemics have resulted from the spillover--the introduction of a novel pathogen into humans from animals--of diseases previously only found in animals. The most well-known of these are the sudden acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) epidemics, the SARS-CoV-2 pandemic, and the ongoing AIDS pandemics [1].
While wild animals are more frequently the source of zoonoses, the animals we have domesticated have their own virulent and devastating diseases [1] [3]. In addition, these livestock pathogens also cause issues for people because of the significant economic losses they can inflict [4] [5]. This problem is exacerbated when the causative pathogens have no vaccine, or treatment, or are under-researched. This is the case for many neglected tropical diseases (NTD) like Rift Valley Fever virus (RVFV) as well as others like Maedi-Visna virus (MVV).
These diseases of livestock also disproportionately impact the livelihoods of people in developing countries. Nearly 1 billion people worldwide derive their income from livestock rearing, and in many African countries affected by livestock diseases like RVF livestock products make up about 30% of national GDP [6].
Rift Valley Fever
Virus
Virion Strucutre and Genome
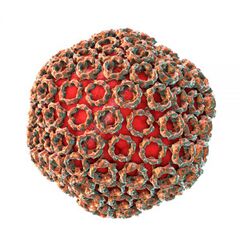
Rift Valley Fever is caused by an approximately 100 nm diameter spherical Phlebovirus in the family Bunyaviridae. It has a negative sense RNA genome with three segments, L, M, and S. The large (L) segment codes for the virus’s RNA-dependent RNA polymerase, and the medium (M) segment codes for the glycoproteins that coat the surface of the viral lipid bilayer envelope [7]. The small (S) segment is ambisense and codes for two proteins: a nucleoprotein and a virulence factor [8]. The nucleoprotein (N) aids the RNA-dependent RNA polymerase in transcription and replication of the viral genome. The virulence factor (NSs) plays a significant role in suppressing the innate host immune response [8]. The NSs factor suppresses the immune system by forming fibrils, clusters of the NSs proteins that appear filamented, which act as a general inhibitor of transcription as well as induce many DNA damage signaling proteins within the cell [9]. This has the effect of halting the cell cycle, typically in S phase, which allows for greater viral replication [8] [9].
Viral Transmission
The virus is also an arthropod-borne virus or arbovirus [10]. For RVF, the virus is vectored by the species in the Aedes and Culex genus. This transmission varies on location but is often spread by the Aedes aegypti mosquito due to the near-global distribution. Additionally, RVFV can be transmitted through the eggs of Aedes aegypti mosquitoes [8][10] . This vertical transmission of the virus from a female mosquito to her offspring plays a significant role in the persistence of the virus. In this way, the mosquito acts not just as a vector of the virus, but also as a reservoir host and can maintain the presence of the virus in the absence of mammals to infect. Additionally, when there are floods, and female Aedes mosquitoes have more opportunities for egg-laying, there are many more cases of RVF in animal and human populations because there is an influx of RVFV positive mosquitoes [10]. The virus can also be spread between animals in contaminated body fluids. These two modes of transmission, by mosquito vector and bodily fluid exposure, contribute to human exposure to the virus.
Animal Health
Symptoms
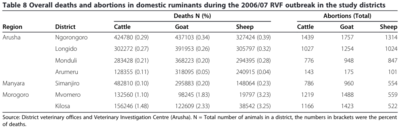
Rift Valley Fever is an endemic disease in much of sub-Saharan Africa as well as Egypt, Saudi Arabia and Yemen. It is caused by the Rift Valley Fever virus (RVFV) and causes hemorrhagic fever. It typically causes nonspecific symptoms but can cause significant morbidity and mortality in sheep and goats [11]. When symptoms do show they present as a biphasic fever of up to 108 degrees F (42 degrees C), malaise and sluggishness, as well as hematemesis, hematochezia, and nasal discharge. The most severely affected individuals are young goats (kids), lambs, and pregnant ruminants [11]. In kids and lambs the mortality rate is between 70-100% [12]. In the adults of the same species the mortality rate is lower, around 10-30%. Cows and calves are affected similarly. Calves infected with RVFV can have mortality rates as high as 70%, with mature individuals experiencing a mortality rate of 5-10%. However, recorded rates of abortion in ewes is as high as 100% whereas sows consistently abort in greater than 10% of cases [5] [11].
Diagnosis
Because the disease is nonspecific, conditional factors can often give an indication that there is an outbreak of RVF. Factors like heavy rainfall, and large mosquito populations act as predictors for outbreaks (figure 4) and significant abortions post infection can be a sign that there is a current outbreak [5] [11]. Tissues from recently aborted fetuses from cattle or sheep contain high titers of the virus and can be used for its isolation. Serum or whole blood samples from animals in the febrile stage of the disease can also be readily used for viral isolation. However, the procedures for viral isolation are slow and expensive so new technologies were vital to accommodate the need for rapid assessment of RVF infection [7]. This came in the form of real-time reverse-transcription loop mediated isothermal amplification assays (RT-LAMP). These RT-LAMP assays had the benefits of high diagnostic accuracy within 30 minutes and are manufactured with relatively inexpensive materials, making it a promising diagnostic tool not only for global health NGOs but also for the people who raise livestock [7].
Treament and Prevention
There is no current treatment for animals with RVF leaving vaccination as the only broadly applicable prevention strategy. There have been several vaccines developed which have seen varying levels of success. The earliest vaccine for RVF was developed in 1949 and is still used today, although its use of a modified live vaccine (Smithburn) retains some virulence and is known to induce abortions and does not produce sufficient antibody response in cattle [7] [11]. More recent attempts at a vaccine have proved promising with a live-attenuated vaccine recently being approved in South Africa and some recombinant vaccines expressing the RVFV surface glycoproteins are in development [7] [10] [11]. However, in most places, RVF does not have long lasting endemic infection and so vaccines are used as an emergency measure in the event of an outbreak [11].
Human Health
Transmission
People most often infected with RVF get it from exposure to infected livestock, although some transmission via mosquitoes does occur [14]. This spillover can occur anytime infected fluids can enter the human body. Infection can happen through cuts on hands or arms from contaminated fluid that splashes a person during births, butchering, or veterinary procedures. The virus can also travel in aerosols which a person from a sick animal can inhale. Given the multitude of ways that RVF can pass from animals to humans, it is important to note that there have been no cases of human-to-human transmission of the virus.
Symptoms
RVF is a generally mild disease in humans with an incubation period of 2-6 days. Typically, people are asymptomatic or have a mild illness frequently including flu-like symptoms such as fever, muscle pains, nausea or vomiting. In less than 10% of people, the disease can progress and present as one of 3 forms: ocular form, meningoencephalitis form, or a hemorrhagic fever form. Both the ocular presentation and the meningoencephalitis presentation occur 1-4 weeks after infection. People who contract the ocular form will have 10-12 weeks of vision impairment and half will have permanent blindness [10] [14]. The second form can lead to severe headaches, and brain damage which is rarely fatal but causes lifelong neurological defects [10] [14]. In less than 1% of patients, the hemorrhagic fever form of the disease has a fatality rate of 50% after 3-6 days due to the blood loss that occurs. In all recorded human cases of RVF, only about 1% of people have died [14].
Economic Impact
RVF outbreaks in Africa are sporadic and generally isolated but can cause significant losses to the people who raise and work with livestock and animal products. These losses can take years to recover from, and extend beyond losing numbers of sheep, cattle or goats. In the 2010 South African outbreak of RVF the sheep farmers lost an estimated 10 million USD worth of livestock [6]. Earlier, the 2007 RVF outbreak in Kenya cost the nation and its farmers close to 32 million USD [15].
Maedi-Visna
Virus

Virion Structure and Genome
The Maedi-visna virus (MVV)(called ovine progressive pneumonia in the US) is the causative agent of Maedi-visna. This virus belongs to the Lentivirinae family in the subclass Retroviridae. MVV and another virus caprine arthritis-encephalitis virus (CAEV) are labeled small ruminant lentiviruses (SRLVs) due to their highly homogeneous genome [3]. MVV primarily infects sheep while CAEV infects goats. However, there are cases of infection of one virus in the other animal, a trait unusual for lentiviruses [16].
The Maedi-Visna virus is a spherical virion with a 3 part genome [17]. The genome is between 8,400 and 10,000 nucleotides long and codes for 3 genes gag, pol, env (common to all retroviruses) as well as its accessory genes. Like other retroviruses gag encodes for the virus’s 3 glycoproteins: proteins for the capsid, for the nucleocapsid, and for a matrix protein which binds together the viral capsid and the viral membrane [17]. The pol gene codes for the RNA-dependent DNA polymerase, a reverse transcriptase, as well as a viral protease, integrase and dUTPase. It is this pol gene that codes the essential functions that allows for viral RNA to DNA reverse transcription and integration into the host genome. The env gene codes for the other set of viral glycoproteins including the surface (SU) and transmembrane (TM) glycoproteins. The SU glycoproteins is what binds to cellular receptors and allows for entry into the cell. It is also the target of antibodies during host immune response. However, its variable genetic structure complicates antibody recognition of virions with varied SUs [16] [17]. The TM glycoprotein has a role in the fusion of the viral envelope and plasma membrane of the host cell [16].
In addition to these conserved proteins, MVV also has a series of auxiliary genes: vif, a vpr-like gene, and rev. The vif gene contributes to host antiviral resistance in other retroviruses like HIV-1, however, its exact role in MVV is still not fully understood [18]. The function of the protein coded by the vpr-like gene similarly is not fully characterized in MVV. It was previously thought to be a tat gene, coding for simulating the expression of viral promoters but more recent studies do not support this early conclusion [17] [18]. Finally, the rev gene is important regulation in viral expression [18].
Viral Transmission
Transmission of MVV can happen vertically or horizontally. Vertical transmission occurs when a ewe passes the virus to her offspring either during gestation (although this possibility is controversial), or from the ewes maternal body fluids or blood during or after birth [4]. Lactogenic transmission of the virus is thought to be the most common route of vertical transmission [4] [19] as MVV has been found to replicate both in the epithelial tissue of mammary glands [4]. Horizontal transmission occurs through aerosolized particles when in close contact with infected individuals but viable viruses have also been found in feces, and fecally contaminated water [4] [19].
Animal Health

Symptoms
Maedi-visna is a viral disease of sheep and goats with a global distribution [4]. Its name stems from its origin in Iceland where maedi refers to the “labored breathing” of the respiratory form visna refers to “wasting” that occurs from the neurological form [21]. As a lentivirus, the replication of the virus within the host is slow, as is the onset of disease. However, once infected, an animal will be a contagious host for life. Unlike other lentiviruses like HIV-1, MMV does not cause immunodeficiency [17]. Instead, Maedi-Visna most commonly presents with progressive weight loss, dyspnea, increased respiratory rate, as well as lameness. The underlying cause of the dyspnea and increased respiratory rate is pneumonia common to MVV infected sheep [4] [16] [17] [20]. This respiratory form is the most common and most deadly. Once symptoms of this respiratory form are observed, the animal likely only has about a year until the progressive lesions in its lungs become too hindering [16] [22]. These lesions are the result of the body's immune response to the viral, as opposed to a result of viral infection in and of itself [22].
The second most common presentation of MV results from infection of the mammary glands. This causes mastitis in sheep within a few days postpartum and can be noticed as hard utters [4]. This is accompanied by decreased milk production [4]. Eventually, the disease can progress in this area as well and form lesions of the mammary gland and stenosis of the milk duct [16] [20].
A final presentation that MV can take is as a neurological form. In this presentation, sheep will suffer from ataxia and paralysis of the hind legs [23].This form often results in the culling of the animal as it no longer has the independence to function [20]. Encephalitis and eventual lesions of the central nervous system also can occur [16].
Diagnosis
Diagnosis is vital for the control of MV. Being a lentivirus, waiting for notable clinical signs of the disease to attempt to curb its spread is not a viable strategy. Fortunately, there are a number of rapid and sensitive tests that can diagnose MVV. The two most commonly used tests for MVV and CAEV are enzyme-linked immunosorbent assays (ELISAs) and agar gel immunodiffusion (AGID) [4] [17] . ELISAs function by detecting antiviral antibodies and improve upon AGID by detecting antibodies at lower concentrations, meaning they can be deployed earlier in the incubation period [17]. Newer methods using monoclonal antibodies or recombinant proteins are also being developed which would be able to detect viral envelope proteins and overall have a greater specificity for detecting MVV and other small ruminant lentiviruses [17]. Another current method of viral detection is the use of PCR to amplify MVV RNA and proviral DNA in a variety of tissues including in milk [4] [17].
Treatment and Prevention
There is no treatment or vaccine for Maedi-visna. Development of a vaccine for MVV is hindered by its broad genetic variability between strains, mechanisms for immunoprotection and antiviral resistance, and need for a high antibody titer to be effective against the virus [4] [17]. Even so, new strategies are being developed that may yet provide a viable MVV vaccine [4].
Given that a significant mode of transmission is through infected milk, lambs born to ewes known to be MVV positive are removed from their mothers and raised in a separate flock [17] Additionally, constant vigilance and testing is needed to watch the spread of disease and prevent infection of MVV free flocks. If an individual tests positive, removal from the flock and isolation should be the first step towards halting the transmission of the disease. In areas with low seroprevalence of the MV, it may be viable to employ culling of all seropositive individuals. However, with greater areas of greater seroprevalence, complete culling may not be the best option [4].
Economic Impact
The exact number of sheep lost to Maedi-visna in any one outbreak or region is difficult to estimate. Nonetheless, seroprevalence studies in some areas indicate their flocks are up to 95% positive for MVV [24]. This may not be true of all flocks of sheep, but for areas where an infected flock is established, the reduction to milk production from the mastitis and the culling that occurs when sheep develop the neurological form has the potential to nearly wipe out entire flocks. This infection presents a problem for sheep farmers, especially those in smaller farms where a MV outbreak may cause the end of their operation.
Human Health
There have been no documented cases of Maedi-visna spilling over into humans [19]. However, Maedi-visna virus and its closely related caprine arthritis-encephalitis virus (CAEV) are lentiviruses in the same genus of retrovirus as HIV-1 and HIV-2. Given that the HIVs originated from simian immunodeficiency virus (SIV) within the last 150 years [2] the potential spillover of MVV or CAEV into humans could be devastating.
Conclusion
Livestock pathogens like the Rift Valley fever virus and the Maedi-visna virus can be devastating to the animals they infect and outbreaks can result in very high morbidity and mortality. In addition, due to the close proximity of infected livestock with their human caregivers, livestock pathogens present a risk for spillover of a zoonotic disease into humans. Spillovers of zoonoses have, in the past, caused epidemics like SARS and MERS, as well as pandemics like SARS-CoV-2 and AIDs. The most effective strategies we have against many of these livestock diseases is surveillance and broad vaccine usage. However, more effective vaccines are needed, as is greater attention to the economic destruction that livestock disease outbreaks like Rift Valley fever and Maedi-visna can cause.
References
- ↑ 1.0 1.1 1.2 1.3 Qiu, Y. et al., "Global prioritization of endemic zoonotic diseases for conducting surveillance in domestic animals to protect public health." 2023. Phil. Trans. R. Soc. B 378.
- ↑ 2.0 2.1 Quammen, David. "Spillover: animal infections and the next human pandemic." 2012. W.W. Norton & Company.
- ↑ 3.0 3.1 Minardi da Cruz, et al., "Small Ruminant Lentiviruses (SRLVs) Break the Species Barrier to Acquire New Host Range" 2013. Viruses 5:1867-1884.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 Kalogianni et al.: Etiology, Epizootiology and Control of Maedi-Visna in Dairy Sheep: A Review. 2020. Animals 10:616.
- ↑ 5.0 5.1 5.2 5.3 Chengula et al.: "Socio-economic impact of Rift Valley fever to pastoralists and agro pastoralists in Arusha, Manyara and Morogoro regions in Tanzania" 2013. SpringPlus 2:549.
- ↑ 6.0 6.1 Mdlulwa, Z. and Ngwane, C. "Evaluating the impact of 2010 Rift Valley fever outbreaks on sheep numbers in three provinces of South Africa" 2017. African Journal of Agricultural Research 12:979-986.
- ↑ 7.0 7.1 7.2 7.3 7.4 Pepin, M. et al. "Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention" 2010. Veterinary Research 41:61.
- ↑ 8.0 8.1 8.2 8.3 Lorenzo, G. et al. "Understanding Rift Valley fever: Contributions of animal models to disease characterization and control" 2015. Molecular Immunology 1:78-88.
- ↑ 9.0 9.1 Baer,A. et al. "Induction of DNA Damage Signaling upon Rift Valley Fever Virus Infection Results in Cell Cycle Arrest and Increased Viral Replication" 2015. Journal of Biological Chemistry 278:10.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 CDC. "Rift Valley Fever" 2023. US dept of Health and Human Services.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Thompson, P. "Rift Valley Fever in Animals." 2022. Merck.
- ↑ World Organisation for Animal Health: Rift Valley Fever.
- ↑ Rostal, M. et al.: Farm-Level Risk Factors of Increased Abortion and Mortality in Domestic Ruminants during the 2010 Rift Valley Fever Outbreak in Central South Africa. Pathogens 2020 9:94.
- ↑ 14.0 14.1 14.2 14.3 "Rift Valley Fever" 2018. World Health Organization.
- ↑ Rich, K. M. and Wanyoike, F.: An Assessment of the Regional and National Socio-Economic Impacts of the 2007 Rift Valley Fever Outbreak in Kenya. The American Journal of Tropical Medicine and Hygiene 2010 83:52-57.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 Gomez-Lucia, E. et al. "Maedi-Visna virus: current perspectives" 2018. Veterinary Medicine: Research and Reports 9:11-21.
- ↑ 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 17.11 Pepin, M. et al. "Maedi-visna virus infection in sheep: a review" 1998. Veterinary Research 29:341-367.
- ↑ 18.0 18.1 18.2 Franzdóttir, S. R. et al. "Two mutations in the vif gene of maedi-visna virus have different phenotypes, indicating more than one function of Vif." 2016. Virology 488:37-42.
- ↑ 19.0 19.1 19.2 [ http://www.cfsph.iastate.edu/DiseaseInfo/ factsheets.php. Rovid, A. "Small Ruminant Lentiviruses: Maedi-Visna and Caprine Arthritis and Encephalitis. ." 2015. Center for Food Security and Public Health.]
- ↑ "Maedi-visna." World Organisation for Animal Health.
- ↑ 22.0 22.1 MacKay, Evelyn. "Lentivirus Pneumonia in Sheep and Goats (Ovine Progressive Pneumonia, Maedi-Visna, Zwoegersiekte, La Bouhite, Graaff-Reinet Disease)." 2022. Merck.
- ↑ Glaria, I. et al.: "Visna/Maedi virus genetic characterization and serological diagnosis of infection in sheep from a neurological outbreak". Veterinary Microbiology 2012 155:137-146.
- ↑ Alba, A. et al.: Seroprevalence and spatial distribution of maedi-visna virus and pestiviruses in Catalonia (Spain)" Small Ruminant Research. 2008 78:80-86.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024
