Malaria (Plasmodium falciparum) in sub-Saharan Africa
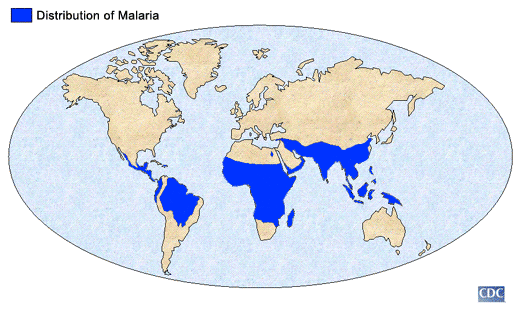
Malaria, especially common in sub-Saharan Africa, is an infectious disease caused by the parasitic protozoan Plasmodium, which can only be transferred by the female Anopheles mosquito. Malaria is spread when the mosquito bites into a person who is already infected. The parasites from the blood uptake reproduce in the mosquito and mix with the saliva so that the next time the mosquito bites another person, parasites are transferred.[1]
There are four parasitic protozoans which cause malaria: Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax. Of these parasites, Plasmodium falciparum is the most dangerous and can cause coma or death.[2] Symptoms include high fever, chills, vomiting, and nausea and they don’t appear until 10-15 days after the initial mosquito bite.[3]
Malaria, which is Italian for “bad air,” has been infecting humans since the last Ice Age, more than 20,000 years ago in sub-Saharan Africa and the Mediterranean region.[4] It was first documented in Chinese medical scripts in 2700 BCE and later in Greece in the 4th century BCE. It was then that Roman writers attributed malaria to swampy areas. The discovery of a tree bark that remedies malaria was discovered in the early 17th century – Spanish missionaries in South America were introduced to the bark by local Indian tribes to cure fevers. The medicine in the bark, now called quinine, is currently being used as antimalarial drug.[5]
The World Health Organization estimates that 300-500 million cases of malaria occur annually worldwide and more than one million victims are killed every year. About 90% of these deaths are from sub-Saharan Africa, occurring mostly in children under the age of 5.[6] 50,000 deaths occur in Zambia alone. 1 in every 5 children in Africa die from malaria, and costs Africa’s gross domestic product approximately $12 billion per year in lost wages due to debilitating effects of the disease.[7][8] Although malaria is preventable and curable, misuse of antimalarial drugs over the past century led to parasitic resistance to these malaria medications.[9]
Genome of Plasmodium falciparum
There are four species of genus Plasmodium that infect humans; Plasmodium falciparum being the most lethal and virulent. P. falciparum is characterized as a protozoan, a single celled eukaryotic organism. Comparative genome analysis amongst eukaryotes revealed that it is more similar to Arabidopsis thaliana than to any other taxa. Furthermore, 237 P. falciparum proteins strongly matched to proteins in all completed eukaryotic genomes but no matches against prokaryotic proteome, suggesting eukaryotic cell lineage. The proteins that were used for comparison included ones that had contributed to cytoskeleton construction and maintenance, cell cycle regulation, translation, replication and many other functions. These proteins were thus used to differentiate between eukaryotes and prokaryotes.
Using a whole chromosome shotgun sequencing strategy, the nuclear genome of P. falciparum 3D7 was sequenced. It is composed of 22.8 megabases (Mb), distributed among 14 chromosomes ranging from 0.643 to 3.29Mb. 80.6% of the genome is adenosine and thymine rich while 20.4% is guanine and cytosine rich. This percentage increases to about 90% in introns and intergenic regions. It is predicted that 54% of P. falciparum genome are introns. Excluding introns, the mean length of the genome is 2.3kb. Many of the large genes present in the genome do not possess recognizable signal peptides thus cannot be identified. With the presence of large genes, more research needs to be done to identify these genes that encode for proteins that do not possess recognizable signal peptides. Also, centromeres have not been identified.
On the contrary to most other eukaryotic organisms, the P. falciparum genome does not carry long tandem repeated arrays of ribosomal RNA (rRNA) genes. Instead, numerous single 18s-5.8s-28s rRNA units are present on the 14 chromosomes. Unlike many other eukaryotes, the P. falciparum genome does not contain long tandem repeated arrays of ribosomal RNA (rRNA) genes. Instead, Plasmodium parasites contain several single 18S-5.8S-28S rRNA units distributed on different chromosomes. The relevance of this is that the sequence encoded by one rRNA in one unit differs from another unit, thus revealing regulatory development, which can lead to different expression of rRNA that alter cell growth and patterns of cell development. For example, the S-type rRNA gene is expressed in the mosquito vector while the A-type rRNA is expressed in the human host.
Chromosome length is further varied by the subtelomeric regions. Subtelomeric regions are proving to be important in research due to the fact that they exhibit antigenic variation. Telomeres are important in P. falciparum because they inhabit genes necessary for immune evasion. One of those important gene families is termed as var which encodes for the erythrocyte membrane protein which are exported to the surface of the infected red blood cells. This mediates adhesion to host endothelial receptors, causing the separation of infected cells from different organs. This is important to the virulence and to the development of severe disease. The other two gene families are rif (repetitive interspersed family) and stevor (sub-telomeric variable open reading frame).
Of the 5,268 predicted proteins, 733 were identified as enzymes important for chemical reactions and 208 genes are known to be involved in the evasion of the host immune system. On the other hand, about 60% of the proteins do not show similarity to proteins of other organisms thus underlying these proteins to be purely unique. This is a high proportion not observed in other eukaryotes. Still, these differences do not necessarily imply that fewer malaria genes are involved in these processes, but highlight areas of malaria biology where knowledge is limited. [10]
Transmission of Plasmodium falciparum into the host cell
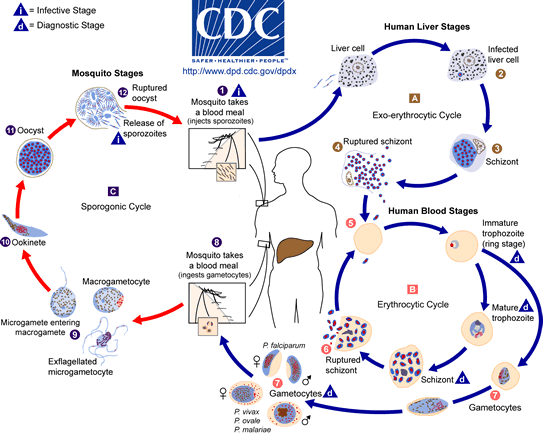
Most commonly, anopheles mosquitoes are the vectors for transmission of Malaria. Half of the life cycle of the protozoan Plasmodium develops in the female anopheles mosquitoes while the other half in the mammalian host after transmission. The transmission of the infectious parasite from mosquito to human relies on the female Anopheles mosquito due to their need to feed on blood whereas male Anopheles mosquito feed on plant-like material.[11] When the female Anopheles bites a mammalian host, sporozoites are rushed into the bloodstream and are directed towards the hepatocytes of the liver. Upon infection, sporozoites develop into merozoite daughter cells.[12]
Sporozoites require multiple mechanisms and proteins in order to evade the host’s immune system. Proteins such as circumsporozoite protein (CSP) and sporozoite surface protein 2 (SSP2) are found in high concentration on the surface of the sporozoite, which help the sporozoite target the liver cells.[13][14] The initiation of asexual Plasmodium developmental begins during the first twenty-four hours of infection. The initial stage (ring stage), the sporozoite envade the human red blood cells (RBCs) and the parasite develops in a parasitophorous vacuole (PV).[12] The next stage of parasitic development is the trophozoite stage where the parasite modifies the RBCs allowing it to transport molecules in and out of the cell, prepare the surface of the red blood cell to mediate cyto-adherence and to digest the cytoplasmic contents – hemoglobin in its food vacuole.[12] For this reason, RBCs are a target by Plasmodium. The Schizont stage is the final stage of development of the asexual daughter cell called the merazoite. Once the merazoite has developed, the PV ruptures first, following rupture of the plasma membrane of the RBC allowing the merazoite to exit and infect the host.[15] However the signal or trigger for merazoite release from the hepatocyte is still unknown.[15]
In a recent study of P. berghei, merosomes which are filled with merozoite-filled vesicles, are budded off from the cell membrane rather than being released upon rupture of the PV and cell membrane of the hepatocyte.[15] Since the host cell has apoptotic features, the parasite is able to manipulate the host cell and prevent phagocytosis of the infected hepatocyte. By having this characteristic, the merazoites are now able to enter into the bloodstream without being attacked by the host’s immune system and cause malaria symptoms to arise.[15]
Transmission of Plasmodium falciparam from host cell to mosquito

The transmission of Plasmodium from human to mosquito requires infectious gametocytes that reside in the capillaries of the inner organs of the infected human.[16] Gametocytes will only appear 3 to 10 days after the merazoites has entered into the bloodstream. However the length of time depends on the strain of the Plasmodium.[16] Once the female mosquito bites and sucks the blood from the infected host, only then do you see the formation of male and female gametocytes fusing to produce thousands of sporozoites. They are infectious once they migrate to the mosquito’s salivary glands. Then the infected female mosquito will again find another host to feed off its blood and inject the infectious sporozoites to the host.[11] Now the vicious cycle of infection has started all over again.
Drug Treatment & Resistance
One of the drugs that are used to treat malaria is chloroquine. Currently this is one the main drugs in the market alongside with Artemisinin. The main use of the chloroquine drug is to inhibit hemoglobin degradation that is seen in the trophozoite stage of development. By inhibiting degradation, the trophozoite is not able to use hemoglobin as a food source, thus eventually causing cell death. However due to the parasites rapid resistant mechanism toward the drug, chloroquine is seen to accumulate in the digestive vacuole making it ineffective towards the parasite. Resistance of the drug was initially attributed towards alterations in the copies of the gene called pfMDR1 and was combined with isolated mutations. Other theories of resistant include a change in the pH gradient, or an alteration in membrane permeability or possibly both. There has also been a mutation of the PfCRT gene that codes for transmembrane protein on the digestive vacuole membarane conferring chloroquine resistance. A drug called Verapamil is being studied to reverse chloroquine resistance. Through this drug, a new membrane protein has been identified, Pgh1. Altered Pgh1 has shown to affect the transport of chloroquine.[17]
Chemoresistance is another hurdle that researchers are facing. Chemoresistance refers to the resistance caused by using multiple antimalarial drugs. By combining several drugs and using it in mass quantities, simultaneous resistance can occur. The mechanisms by which parasites become chemoresistance are by chromosomal mutations. Looking from a genetic standpoint, malaria is caused by three different groups – humans, anopheles (mosquitoes) and Plasmodium. One can thus imagine the different mitotic mutations that can occur while the Plasmodium cells are replicating in the anopheles and in the humans and the meiotic recombinations that can occur just after the male and female gametocytes fuse together. Secondly, mosquitoes can carry different strains which is a problem because recombination of two different strains can occur causing a more resistance and possibly lethal strain. Thus highlighting the diversity of P. falciparum and the struggle to find an active drug to fight against the malaria parasite.[17]
Currently, the world health organization recommends Artemisinin-based combination therapy (ACT) as a first-line drug to treat uncomplicated malaria. Artemisinin is a sesquiterpene lactone, derived from a plant that is grown in China and was used in traditional Chinese medicine. Although the exact mechanism of how the drug kills the parasite is still not fully understood. It is thought to be an attack on a protein associated with the parasite. Nevertheless, studies have shown the drug acts very rapidly once inside the blood stream. Parasites are quickly eradicated from the body and have gametocytocidal properties. In turn, it reduces the number of gametocytes, reducing the toxicity of the bacteria and thus the transmission of the parasite to the host cell. Due to its short half life, resistance is more likely. For that reason, a combination therapy is being used which is showing promising results. But recent in vivo studies have shown some resistance to the drug but more studies still need to be done.[18]
Drug resistance is not a new issue that researchers are facing with. Combined use of chloroquine, amodiaquine, quinine, and other drugs have been used since the 1950s resulting in the development for resistance. Resistance outside of Africa is a lot higher as seen in the areas of Thailand, Myanmar and Cambodia. The overall prevalence in Africa to chloroquine resistance is 50% but this percentage has not increased in the last decade. But resistance to quinine, mefloquine and halofantrine is not high and low levels are still being used in remote areas in Africa. What studies do show that although levels of malaria are not as high compared to other Asian countries, the issue over multiple drug resistance is compelling and is ever more essential to find a solution before resistance spreads.[18]
Why is this disease a problem in sub-Saharan Africa
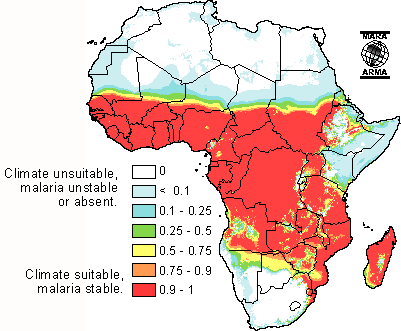
Environment and Lifestyle
Sub-Saharan Africa is a major site of malaria transmission due to is geographical location in the tropic zone. The tropical areas have large amounts of rainfall which create vast breeding grounds to allow the development of egg to larvae to pupae to adult. After a mosquito has developed and become infected with the parasite, the warm climate of sub-Saharan Africa allows the rapid development of the parasitic growth cycle (extrinsic cycle) to occur. The warm temperature actually condenses the length of parasite development permitting rapid transmission. Additionally, due to the warm climate, many sub-Saharans sleep outside during good weather more often allowing the potential to be bitten to increase substantially. This is especially the case among agricultural workers who often sleep in close proximity to fields during the harvest without the protection of insecticide bed nets.[19]
Urbanization and Economics
Today, sub-Saharan Africa is the fastest urbanizing continent. More than ever, human migration patterns are drawing populations closer together in large urban city centers. As of 2003, roughly half of the population south of the Sahara desert lives in urban areas. This increase in proximity and density of populations in sub-Saharan Africa has increased the spread of malaria dramatically. Hospitals in sub-Saharan Africa report 15% of admissions are due to malaria. However, much of the urbanization that has taken place is a migration of the rural poor to the city centers. Urbanization has been coupled with poor public sanitation, meager sources of public water, and low grade housing construction. In sub-Saharan Africa, urbanization is not a model of the typical western design. In many urban areas, the main business hub or downtown area is the only place where working water and sewage systems are accessible. Poor shanty towns and farms are located side by side next to affluent neighborhoods. Livestock animals are herded through streets which increase the low level of public sanitation. The rise in urban farming and construction provides generous water sources for mosquitoes to breed alongside construction sites. Travel among individuals who visit rural areas for labor and return home to urban centers also increases the transmission rate. Furthermore, lack of education and financial resources advances transmission. Those that fall in this category of low socio economic status have lower quality housing and lack factors that prevent the spread of malaria such as proper household sanitation, screens on doors to prevent mosquitoes from entering the home, bed nets which provide protection while sleeping, indoor insecticide spraying, and prophylaxis such as chloroquine.[20]
Drug Resistance
Another factor that has been in development since the late 1950’s is the increased resistance to many antimalarial drugs. There are two major classes of antimalarial drugs which consist of the anti-folates and quinoline containing drugs. These drugs affect the asexual erythrocytic stage of the parasite as the means of attack. One of the most common drugs used to combat malaria is chloroquine. Chloroquine is used because it only costs about $0.08 USD per treatment and is readily available. Resistance is on the rise in sub-Saharan Africa because of inability of those infected to finish a complete dose due to factors such as ill side effects, lack of food to consume the medicine with, and lack of knowledge about resistance. Additionally, how frequent an individual becomes reinfected after treatment also increases resistance.[21]
Political Issues
From the 1940s to 1970s global efforts to combat malaria were strengthened in the region of the subtropics (region north and south of the tropics) and diverted in the tropics (region including sub-Saharan Africa) because of the ease of control in the subtropics region. Partly due to developing resistance to DDT (dichlorodiphenyltrichloroethane, pesticide used to combat mosquito breeding grounds) and chloroquine (common first line of defense of malaria used by humans) efforts to eradicate malaria were abandoned in sub-Saharan Africa. In many of the subtropics region, malaria control/elimination was achieved in Asia, Latin America, southern Soviet Union, southern Europe, and in the southern United States. In the 1970s, the U.S. withdrawal from Vietnam further deemphasized the malaria epidemic since U.S. troops were no longer fighting in the region. Sub-Saharan Africa was not politically on the map for large amounts of foreign aid or intervention which it desperately needed. During the 1980s, much of the global campaign on malaria ceased due to the virtual elimination from the subtropics region and much of the foreign aid to combat the disease was dramatically reduced. Additionally, during the 1980s, many of the sub-Saharan countries fell into debt and the foreign governments and international institutions which served as creditors for these African nations began introducing national budget cuts in order to recover credit and loans. Such institutions were the International Monetary Fund and the World Bank which served as creditors. With already meager resources available to fight the spread, public health deteriorated coupled with extreme population growth, resistance to drugs and insecticides, as well as urbanization. In the 1990s, the World Bank did not make a single loan to any sub-Saharan nation to combat malaria. The only two loans made to fight malaria during the 1990s were to India and Laos. Additionally, many U.S. pharmaceutical companies did not develop new anti-malarial drugs or vaccine research since many American and European travelers were not traveling to sub-Saharan African; hence, the small market profitability. Recently, efforts have been made by the World Health Organization, Global Fund to Fight AIDS, TB, and Malaria, among others but lack substantial financial backing to implement broad global action against this epidemic.[22]
What is being done to address this problem

Currently malaria is a worldwide problem in which many worldwide organizations and the United States have partnered together to fight against the spread of malaria. [23] In particular, in sub Saharan Africa, the World Bank instigated the Booster Program for Malaria control. This program began in 2005 with the goal to halve the burden of malaria in 20 focus countries in Africa by 2010. This program is based on providing prevention and control strategies to the local people. One method that has proven effective is the insecticide- treated net. Statistics have shown that if 75% of the people in a community were to use insecticide-treated nets properly, mosquito population drops by 90%, transmission is reduced by 50% and child deaths are reduced by 20%. [23] However, affordability is the real problem. Funding for the program comes through sponsorships from companies and other governments. For example, Exxon Mobile has donated a lot of funds to support the Booster Program in Nigeria. The US government, World Health Organization, and United Nations Children’s Fund (UNICEF) are other major contributors to this program. [24]
While in the US, former President George W. Bush, extended the President’s Malaria Initiative (PMI) through 2010 to coincide with the Booster Program. This is a 9 year program to halve the problem of malaria in 15 focus countries in Sub- Saharan Africa. This funding goes to insecticide treated mosquito nets, sprays, preventative treatments for pregnant women, and development and distribution of artemisinin based combinational therapy. [25] Alongside with the PMI and Booster program, the Global Fund to fight HIV, TB, and Malaria program is another initiative that helps allocate and utilize the funds appropriately. In the past, the Global Fund has sponsored distribution of 88 million bed nets, improvement in infrastructure, and training of individuals to care for those with diseases especially in Sub -Saharan Africa. In a continuing effort, the US has donated about $15.6 billion to the Global Fund in order to further sponsor programs fighting the spread of malaria, TB, and HIV in affected countries. [26]
Medicines for Malaria Venture (MMV) is another program initiated by the Swiss foundation aimed at developing and distributing antimalarial drugs. They hope to lessen the burden of malaria through innovative medicines aimed specifically at young children and expectant mothers who are in very high risk groups. They also are developing other combinational therapies such as the ACT as previously described. [27]
What else could be done to address this problem
Many of the techniques that can be done to control the spread of malaria focus on preventing infection, rather than treatment of infected individuals. These pre-infection methods include development of a vaccine, preventing mosquito larvae growth, and killing adult mosquitoes that may be infectious. Post-infection methods include halting the distribution of counterfeit drugs and early diagnosis of the disease. Although there are many potential strategies to handle the issue, they’ve been difficult to implement for various reasons.
Vaccines
Although vaccination would successfully reduce and stop the spread of malaria, there is currently no complete effective vaccine that has been developed. The underlying problem in all antimalarial medication is that the parasite and the mosquito both evolve quickly, which then makes the vaccine ineffective in the future.
The genome of Plasmodium falciparum was sequenced by Dr. Stephen Hoffman in 2002, which gives us a greater understanding of possible components which the medication can target.[28] This research was done in support of earlier studies where mice were immunized with inactive or dead sporozoites, which is the form of the cell that infects new hosts.[29][30] Proteins, namely the circumsporozoite protein, on the surface of the sporozoites are believed to be recognized by the body’s immune system; hence most malaria vaccines are created around this protein.[31] The RTS,S/AS02A vaccine, created around the circumsporozoite protein, was developed and tested on 2,000 Mozambican students in October 2004 with optimistic results – the vaccine decreased the risk of infection by 30% and reduced the severity by over 50%.[32] In October 2007, 214 Mozambican infants were treated with the second trial of RTS,S/AS02A vaccine with even more positive results – the vaccine reduced risk of infection by 62%.[33] However, much improvement, research, and testing still need to be done to have this vaccine available on the market.
Education
Malaria education in Africa is not emphasized enough, which results in poor community involvement and acceptance of various control strategies.[34] The Roll Back Malaria initiative created by WHO acknowledges that community-based effort is essential to combat malaria, but even with this program set in place, much more needs to be accomplished in order to educate the public in malaria awareness.
Since malaria is especially prevalent among children, extra precautionary measures should be taken with sanitation in schools. Along with this, schools should provide children with health education. FRESH is a program founded by the United Nations Educational, Scientific, and Cultural Education (UNESCO) with the purpose of reducing and preventing malaria through schools. This is achieved by a providing clean, sanitary environment, free of stagnant water which serves as breeding grounds for mosquitoes; thorough education which prepares children to be aware of the disease and to react appropriately if they or someone else is sick; health services to both students and faculty; and community outreach.[35] This community-based approach towards education in both health and sanitation can greatly reduce malaria infections.
Prevention
Recently, researchers have been exploring the potential benefits of stopping mosquitoes before they get a chance to infect humans. If the mosquitoes were to be stopped before they got a chance to infect humans, it could help slow the progression of malaria.
Brian Foy, an assistant professor at Colorado State University, has been testing a new method to kill the bugs during the 10 to 14 days it take malaria to develop inside a mosquito before it is transmittable to another person. He tracked mosquitoes and found that those that had bitten people taking medication to combat river blindness, died for up to a week, successfully stopping two infections at once. [36] Upon contact with the blood, the mosquitoes died. The problem with this include making sure the mosquitoes receive a high enough dosage of the drug, but not develop resistance to it, as well as having the mosquitoes bite while the drug is still in the bloodstream.
Other methods of stopping the mosquitoes before they get the opportunity to infect others was developed by Szabolcs Marka, assistant professor at Columbia University, involving lasers.. He studied the potential use of a 'laser barrier' around a house or village that could detect the mosquitoes within range, and disrupt their sensory networks, rendering them unable to locate their human prey [37] This method would be difficult to implement financially but has proven to be an effective way to avoid mosquito bites and malaria infection.
Elimination of Counterfeit Drugs
Counterfeit antimalarial drugs are on the rise in Africa, as well as other countries. Rather than helping those taking the medications, these drugs are actually threatening the lives of hundreds of thousands of people by increasing the number of drug-resistant strains of the disease, making it harder for drug companies to develop efficient drugs to treat them. The main concern isn't that these counterfeits don't have the correct ingredients in them, but that there are only trace amounts of the active ingredient. Without the correct dosages, these medications that should be stopping the spread, are allowing the disease to develop resistance more easily. The high cost of artemisinin-based combination therapy and their limited supply to those that need it, is also providing 'a favorable situation' for the market of the counterfeit drugs to spread. [38] Regional and national approaches are necessary in order to combat the issue, including law enforcement and drug registration and inspection.[39] It is also crucial that public awareness be heightened among both consumers and health care providers. In order to effectively stop the spread antimalarial drug resistances, these fake drugs need to be eliminated and proper antimalarial drugs must be used properly and made available to those in need. However, a reliable method to detect these counterfeits without lab tests has not been found.
Early Diagnosis

Because antimalarial drugs and vaccines are so expensive and limited, it’s important to invest money into diagnostic products that are effective and accurate. Misdiagnosis of malaria leads to too many prescriptions of limited drugs and vaccines. In the past couple of months, 30.8% of the patients were misdiagnose and 29.3% were given drugs they didn’t need. With microscopy and the rapid diagnostic test (RDT), patients can be diagnosed accurately. Diagnosis of malaria is 93.5% accurate with microscopy and 97.5% accurate with RDT [40]. RDT detects the parasitic antigens (histidine-rich protein-2 (PfHRP2)) [41]that are present in malaria quicker and more accurate than microscopy [42]. Although RDT is more expensive than microscopy, it needs to be implemented in areas that have severe cases of malaria. Since the diagnosis are quick and accurate, treating patients with malaria will be more cost efficient and there will be more vaccines and anti-malaria drugs to be used efficiently. Overtime the money saved on misdiagnosed patients will cover the costs of RDT [43]. In the absence of microscopy and RDT, children five and younger and pregnant women with high fevers are automatically given anti-malaria drugs because they are more prone to malaria. [44]
Pesticides
A method used to control malaria in India during the late 1940s was spraying a pesticide, DDT, throughout the country. It was a huge success, but there were many side effects that put everyone’s lives in danger. Many people died from eating food that was contaminated with pesticides. Some of the other health effects for consuming pesticides are the immune system weakening, problems with the hormones, losing intelligence, birth defects and cancer. [45] Other ecological effects caused by DDT were the thinning of the egg shells and the adult birds would crush their eggs. Also, the pesticide would kill off the cats which increased the number of rodents everywhere. [46] Thus, it became unethical and the ban of spraying pesticides went into effect. Once the use of pesticides was banned in the 1970s, the mosquitoes evolved rapidly to resist the pesticides and there were an outburst of malaria cases. [47]
Sickle Cell
Another way to eradicate malaria is by the use of sickle cells. The problem with using sickle cells is that some believe its morally unethical to induce sickle cells into everyone infected with malaria. Sickle cell individuals are immune to malaria because their HBB gene is mutated which is used for encoding the hemoglobin in red blood cells. The malaria parasite lives in the red blood cells, but if the cells are sickle shaped, the parasitic gene won’t be able to survive.[48]
Summary
Recently malaria has gained resistance to many of the antimalarial drugs that are out in the market. By sequencing the genome, researchers have been able to get a better understanding of the protozoan and the proteins associated with it. By identifying the key proteins associated with parasite development, researchers have been able to attack those proteins and inhibit activity. But even with the antimalarial drugs, societal problems still exist that contribute to the spread of malaria and causing resistance. More focus needs to be put on prevention and how to provide the basic needs such as clean water, food and a shelter. Moreover, many resources are scarce such as adequate medical equipment and the manpower. Action from all levels of the community and government is needed to initiate community programs that can benefit the communities’ infrastructure and the well being of the people. Although issues remain regarding malaria, international organizations have taken leap by creating programs to help alleviate the spread of malaria. Within time and with the help of research, malaria will be eradicated and contained.
References
[1] "WHO | Q&A: malaria." WHO | World Health Organization. Web. 18 Aug. 2009.
[2] "WHO | Malaria." WHO | World Health Organization. Jan. 2009. Web. 18 Aug. 2009.
[3] "WHO | Malaria." WHO | World Health Organization. Web. 18 Aug. 2009.
[7] "Africa - Striking Back at Malaria in Sub-Saharan Africa." The World Bank. Web. 18 Aug. 2009.
[9] "WHO | 10 facts on malaria." WHO | World Health Organization. Web. 18 Aug. 2009.
[16] “WHO: FAQ about malaria.” WHO World Health Organization. Web. 18 Aug. 2009
[23] “Malaria Statistics.” Nets for Life. August 25, 2009.
[24]"Striking back at Malaria in Sub Saharan Africa". The World Bank. August 25,2009.
[25] "Saving Lives in Africa". President's Malaria Initiative. August 25,2009.
[26] "The Global Fund". The Global Fund. August 25,2009.
[27] "Curing Malaria Together". Medicines for Malaria Venture. August 25,2009.
[34] [Narayan R, Sehgal PN, Shiva M, Nandy A, Abel R, Kaul S: Towards an appropriate malaria control strategy: Issues of concern and alternatives for action. Voluntary Health Association of India/Society for Community Health Awareness, Research and Action, New Delhi; 1997.]
[35] “Focusing Resources on Effective School health” UNESCO. Web. 27 Aug. 2009.
[37] Scott, Chris. “Grand Challenge: End Malaria Deaths.” One International. 23 April 2009. 27 Aug 2009
[39] "WHO | Malaria Drug Resistance." WHO | World Health Organization. Web. 27 Aug. 2009.
[42] “WHO| What is an RDT” WHO| Diagnosis, Web. 21 Aug. 2009.
Edited by Andy Chen, Renu Gaur, Matthew Hsia, Alice Nguyen, Amreeta Panesar, Kris Vasant, and Chirag Yadav, students of Dr. Rachel Larsen at University of California, San Diego.
