Meromictic lakes: Difference between revisions
| (35 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Image:Cadagno.jpg|thumb|300px|right|A meromictic lake in Switzerland. Notice how small the area is. In order for this lake to have a layer that does not mix, it must be very deep. This image comes from Wikipedia.]] | |||
[[Image:Cadagno | |||
==Introduction== | ==Introduction== | ||
The | The Encyclopedia of Inland Waters defines meromictic lakes as stratified lakes that consists of two layers that do not completely mix. The top layer, the mixolimnion, is a portion of the lake, usually ending a couple of meters under the surface of the lake, that is exposed to the atmosphere. The mixing of the mixolimnion usually occurs for the majority of the year, especially in the northern latitudes where the mixolimnion does not stratify based on temperature. The one part of the year where these northern meromictic lakes' mixolimnions do not mix is during the winter when ice is covering the surface of the lake. The southern meromictic lakes may have stratified mixolimnions based on temperature and ultimately water density. These mixolimnions may not fully mix during the summer, but they will mix completely from late fall through early spring. The bottom layer, the monimolimnion, is cut off from the atmosphere by the mixolimnion. These two layers are separated by either a thermocline or a chemocline (Water Quality Assessments). The Encyclopedia of Inland Lakes further discribes this separation. A thermocline exists when there is a temperature difference between the surface and bottom of a lake. This temperature difference causes a difference in water density with the heavier cold water near the sediments. This temperature difference holds as long as the temperature of the lake does not cool off. Therefore, meromictic lakes that are stratified by a thermocline are typically found near the tropics where the surface of lakes never freeze. This is not to say that a thermocline cannot separate the mixolimnion and the monimolimnion in temperate climates. However, if this is the case, the lake needs to have a small surface area compared to its depth. This also means that the sides of the lake need to be steeply sloped. The small surface area compared to a lake's depth helps prevent the wind from being able to fully mix the lake when the temerature of the lake is uniform from late fall through early spring. After all, the larger the fetch, the length of a lake exposed to a gust of wind, is , the more likely the lake will turn completely over. This is especially true for shallow lakes with large fetches. The Encyclopedia of Inland Lakes gives a second way for lakes to be stratified into permanently different layers: the presence of a chemocline. A chemocline, in the context of meromictic lakes, is a gradient of salinity. The saltier, and more dense, water would settle to the bottom of the lake therefore forming the monimolimnion. The mixolimnion would have water with significantly less salt dissolved in it. These lakes are somewhat common in deep basins near coastal areas where fresh and salt water interact with each other. | ||
The size of meromictic lakes vary greatly with the largest being the Black Sea | The size of meromictic lakes vary greatly with the largest being the [http://en.wikipedia.org/wiki/Black_Sea Black Sea]. The Black Sea is about 436,400 square kilometers and has a maximum depth of 2,212 meters. This great depth and the fact that the bottom water is more saline than the top is what keeps the Black Sea from fully mixing. Regardless of its size,a meromictic lake is home to a very diverse set of species, especially when it comes to microbial communities. Although individual species do vary depending on which lake is being studied, multiple studies have indicated that there are a few aerobic species at the top of the mixolimnion. Populations and the diversity of the bacteria tend to increase as the thermocline or the chemocline is reached just a couple of meters under the surface of the lake (Ovreas, 1997). This barrier between the two pools of water seems to be the place that has the most amount of microbial species and therefore the highest biodiversity. After all, both aerobes and anaerobes can potentially be within a few meters of each other. Also, like the mixolimnion, light can reach this area, so both photoautotrophs and heterotrophs can potentially exist in this region. Past the chemocline or thermocline is the monimolimnion which is depleted of oxygen. Therefore, only anaerobic bacteria and archaea can function in this zone. Since the monimolimnion hardly ever, if at all, mixes, it is a very anaerobic place where sulfur redox reactions dominate. Methanogenesis, via [[methanogens]], also occurs in the monimolimnion. | ||
==The Environment== | ==The Environment== | ||
| Line 14: | Line 10: | ||
===The Physical Environment=== | ===The Physical Environment=== | ||
====Temperature==== | ====Temperature==== | ||
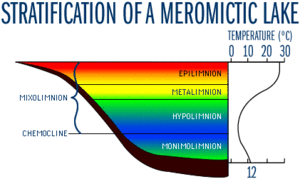
Since most meromictic lakes are deep and have a thermocline, temperature tends to be important. Temperature varies in the different layers of a meromictic lake. | Since most meromictic lakes are deep and have a thermocline, temperature tends to be important. Temperature varies in the different layers of a meromictic lake. The surface of a meromictic lake is the warmest in the summer (Stewart, 2009). In fact, the mixolimnion may be stratified as in the picture to the left. In this depiction, there is an epilimnion, a metalimnion, and a hypolimnion in the mixolimnion. This stratification is caused by varying temperatures with the warmest water being in the epilimnion and the coldest in the hypolimnion. This separation will affect the way the lake mixes during the summer, and more than likely only the epilimnion and the top of the metalimnion will be able to mix well. However, once the colder fall temperatures arrive, this stratification ends up breaking apart, and the whole mixolimnion is allowed to mix entirely again. Regardless, the monimolimnion always remains separate from the mixolimnion, and therefore is not ever in contact with the atmosphere (Stewart, 2009). | ||
====Density==== | ====Density==== | ||
Density is another important physical property in meromictic lakes. Temperature plays a key role in determining the density of the water in the lake. This is why the epilimnion, having the warmer, less dense water, is the top layer of the mixolimnion, and why the hypolimnion, containing the colder, more dense water, is the bottom layer of the mixolimnion during the summer months. Another important factor that affects the density of water is the amount of solutes dissolved in the water, or in this case, the salinity. The more saline the water, the more dense it is. Therefore, very saline water will stay right above the sediments of meromictic lakes. The gradient in salinity is the reason for the chemocline, and is why the hypolimnion in the mixolimnion is not a part of the monimolimnion even though they are roughly the same temperature. This varying in density throughout the meromictic lakes, especially in summer, is what controls most of the chemical processes within the lake. | Density is another important physical property in meromictic lakes. Temperature plays a key role in determining the density of the water in the lake. This is why the epilimnion, having the warmer, less dense water, is the top layer of the mixolimnion, and why the hypolimnion, containing the colder, more dense water, is the bottom layer of the mixolimnion during the summer months. Another important factor that affects the density of water is the amount of solutes dissolved in the water, or in this case, the salinity (Stewart, 2009). The more saline the water, the more dense it is. Therefore, very saline water will stay right above the sediments of meromictic lakes. The gradient in salinity is the reason for the chemocline, and is why the hypolimnion in the mixolimnion is not a part of the monimolimnion even though they are roughly the same temperature. This varying in density throughout the meromictic lakes, especially in summer, is what controls most of the chemical processes within the lake. | ||
===The Chemical Environment=== | ===The Chemical Environment=== | ||
====Dissolved Oxygen==== | ====Dissolved Oxygen==== | ||
Dissolved oxygen is the amount of oxygen that is held, dissolved, in water. | Dissolved oxygen is the amount of oxygen that is held, dissolved, in water. The amount of oxygen that is dissolved in the water is greatest in the epilimnion of the mixolimnion and is lowest in the monimolimnion (Horne,1994). The epilimnion has the greatest amount of contact with the atmosphere and can be thoroughly mixed. This makes it very easy for the oxygen in the atmosphere to dissolve into the epilimnion. Some of this oxygen is able to get mixed in with the top of the metalimnion, but nothing beyond that. Also, since the epilimnion has a warmer temperature than the layers below it, it can hold less oxygen. However, the continuous mixing of the epilimnion during the summer keeps this layer oxygenated. Another way that a lake is able to obtain oxygen is through the photosythesis of plants and phototrophic microbes. Since plants and some microbes need light to survive, they can are limited to the top layers of the lake. These top layers are referred to as the photic zone, and is the area where photosynthesis takes place (Kennesaw State University). The lower layer of the photic zone is where 99 percent of the light from the sun is absorbed by the water. Not all organisms in water contribute to the dissolved oxygen concentration. Some, like fish, heterotrophic bacteria, decomposers, and benthic invertebrates, deplete the oxygen concentration. However, the epilimnion is usually robust enough to support these oxygen utilizing organisms. | ||
The metalimnion is the portion of the meromictic lake that starts to show an oxygen deficiency, especially the lower portion of the metalimnion (The Encyclopedia of Earth). Even though the metalimnion has a cooler temperature than the epilimnion, and can therefore hold more oxygen, it will have a lower dissolved oxygen concentration due to the fact that it is not in direct contact with the atmosphere and cannot be mixed. To add to this lack of oxygen, the aerobic microbes and fish that live in this region of the lake will eventually deplete as much oxygen as they can before they have to move to a different location or die. This is even more true for the hypolimnion and the monimolimnion. In fact, since the monimolimnion rarely, if ever, gets mixed and it does not have organisms that photosynthsize, it usually only has a dissolved oxygen concentration of less than 1 ppm (CWTA). This is far below the amount of oxygen that most fish need to survive. | The metalimnion is the portion of the meromictic lake that starts to show an oxygen deficiency, especially the lower portion of the metalimnion (The Encyclopedia of Earth). Even though the metalimnion has a cooler temperature than the epilimnion, and can therefore hold more oxygen, it will have a lower dissolved oxygen concentration due to the fact that it is not in direct contact with the atmosphere and cannot be mixed. To add to this lack of oxygen, the aerobic microbes and fish that live in this region of the lake will eventually deplete as much oxygen as they can before they have to move to a different location or die. This is even more true for the hypolimnion and the monimolimnion. In fact, since the monimolimnion rarely, if ever, gets mixed and it does not have organisms that photosynthsize, it usually only has a dissolved oxygen concentration of less than 1 ppm (CWTA). This is far below the amount of oxygen that most fish need to survive. | ||
[[Image:Purple_Sulfur_Bacteria.jpg|thumb|300px|right|A lake with a large purple sulfur bacteria population. This image comes from The Friends of Ecological Reserves' website.]] | [[Image:Purple_Sulfur_Bacteria.jpg|thumb|300px|right|A lake with a large purple sulfur bacteria population. This image comes from The Friends of Ecological Reserves' website.]] | ||
==== | ====Redox Gradient==== | ||
Lakes that are stratified, such as meromictic lakes, tend to have a redox gradient between the epilimnion and the hypolimnion, or in the case of meromictic lakes, between the mixolimnion and the monimolimnion (Sondergaard, 2009). This is especially true in eutrophic lakes due to the rapid depletion of oxygen in the summer due to decomposition. The presence of oxygen in the mixolimnion keeps the redox potential higher, and therefore, oxygen is used as a terminal electron acceptor for microbes. However, the deeper water in meromictic lakes has a lower concentration of dissolved oxygen, and therefore a lower redox potential. For instance, water with a dissolved oxygen concentration of 0.1mg/L has a redox potential of 200mV (Sondergaard, 2009). Once the oxygen is used up in an area that does not get readily supplied with oxygen, starting at the thermocline or chemocline, microorganisms have to switch electron acceptors. The next most energy favorable terminal electron acceptors include, but are not limited to, nitrate, manganese, iron, sulfate, and carbon dioxide. These are listed in order of most to least energetically favorable. | |||
This redox gradient also occurs in the sediments of meromictic lakes (Sondergaard, 2009). If oxygen or nitrate is present in the sediments, they are usually in the topmost layer, which tends to be a brown color. A green layer is directly below this followed by a black horizon formed by iron and sulfate reduction respectively. The bottom most layer is usually a grey layer where [[methanogens]] are actively reducing carbon dioxide to methane. Redox gradients may also be seen in [[flooded soils]]. | |||
==Microbial communities== | ==Microbial communities== | ||
===The Mixolimnion=== | ===The Mixolimnion=== | ||
The mixolimnion, since it can act like an individual lake and stratify, and since it has relatively low salinity in terms of the chemocline, can be home for numerous microbial communities that are present in freshwater lakes. These include, but are not limited to algae, actinobacteria, betaproteobacteria, alphaproteobacteria, gammaproteobacteria, cyanobacteria,and various types of freshwater, aerobic archaea. These microbial communities have adapted to use oxygen as their terminal electron | The mixolimnion, since it can act like an individual lake and stratify, and since it has relatively low salinity in terms of the chemocline, can be home for numerous microbial communities that are present in freshwater lakes. These include, but are not limited to algae, actinobacteria, betaproteobacteria, alphaproteobacteria, gammaproteobacteria, cyanobacteria,and various types of freshwater, aerobic archaea (Yannarell, 2009). These microbial communities have adapted to use oxygen as their terminal electron acceptor in order to make as much energy, ATP, as they can. They also selected for living in open water with very little in terms of cover from predators. This means that they had to adapt in order to survive a predator's attack. Some of these adaptations include flagella, binding to larger particles, selecting for a larger or smaller cell body size, and working together as a community like algae do in algal mats. | ||
===The Chemocline=== | ===The Chemocline=== | ||
The chemocline has the potential for the greatest amount of species diversity (Ovreas, 1997). Both fresh and saltwater microorganisms can survive within feet of each other in this layer, and so can aerobic and anaerobic microbes. The chemocline is also the area where the green and purple sulfur bacteria can survive. These bacteria need sulfur and light in order to conduct photosynthesis. This is not to say that these sulfur bacteria cannot survive in the mixolimnion, but rather, they have a greater population near the chemocline. The major adaptation for the microbes that exists in and near the chemocline is that they have to be metabolically diverse. In other words, facultative aerobes and anaerobes have the greatest chance of surviving near the chemocline. This is because oxygen concentrations have a tendency to fluctuate, especially during the summer months when the mixolimnion is stratified. | This zone of rapid change in salinity is in most meromictic lakes. However, there are a few rare meromictic lakes that do not have this chemocline and thus lack the microbial diversity of this section. The chemocline has the potential for the greatest amount of species diversity (Ovreas, 1997). Both fresh and saltwater microorganisms can survive within feet of each other in this layer, and so can aerobic and anaerobic microbes. The chemocline is also the area where the green and purple sulfur bacteria can survive. These bacteria need sulfur and light in order to conduct photosynthesis. This is not to say that these sulfur bacteria cannot survive in the mixolimnion, but rather, they have a greater population near the chemocline. The major adaptation for the microbes that exists in and near the chemocline is that they have to be metabolically diverse. In other words, facultative aerobes and anaerobes have the greatest chance of surviving near the chemocline. This is because oxygen concentrations have a tendency to fluctuate, especially during the summer months when the mixolimnion is stratified. | ||
===The Monimolimnion=== | ===The Monimolimnion=== | ||
In the deepest part of meromictic lakes, anaerobic bacteria and archaea dominate. There is little to no oxygen, therefore microbes will have to find the next best terminal electron acceptor. SRB,some green and purple sulfur bacteria, and methanogens rule this portion of the lake. The main adaptation to living in this portion of the lake is that the microbe needs to be able to use sulfur or carbon dioxide in redox reactions in order to sustain their energy need. | In the deepest part of meromictic lakes, anaerobic bacteria and archaea dominate. There is little to no oxygen, therefore microbes will have to find the next best available terminal electron acceptor. SRB,some green and purple sulfur bacteria, and [[methanogens]] rule this portion of the lake. The main adaptation to living in this portion of the lake is that the microbe needs to be able to use sulfur or carbon dioxide in redox reactions in order to sustain their energy need. | ||
==Microbial processes== | ==Microbial processes== | ||
| Line 50: | Line 44: | ||
<br><b>Photosynthesis:</b> 6CO<sub>2</sub>+6H<sub>2</sub>O+light=C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>+6O<sub>2</sub> | <br><b>Photosynthesis:</b> 6CO<sub>2</sub>+6H<sub>2</sub>O+light=C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>+6O<sub>2</sub> | ||
<br><b>Respiration:</b> C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>+6O<sub>2</sub>=6CO<sub>2</sub>+6H<sub>2</sub>O | <br><b>Respiration:</b> C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>+6O<sub>2</sub>=6CO<sub>2</sub>+6H<sub>2</sub>O | ||
===The Monimolimnion=== | ===The Monimolimnion=== | ||
<br><b>Purple and Green Sulfur Bacteria Photosynthesis:</b> H<sub>2</sub>S+CO<sub>2</sub>=CH<sub>2</sub>O+H<sub>2</sub>O+2S | ====Sulfur==== | ||
Sulfur is a critical element starting at the chemocline and extending down to the monimolimnion. Since there is very little, if any, oxygen in these meromictic layers, the bacteria must find another terminal electron acceptor. Sulfur reducing bacteria, SRB, reduce elemental sulfur to sulfide (Kondo,2006). The other side to this redox reaction is that they oxidize organic compounds like acetate and succinate to carbon dioxide. This sulfur redox reaction can be done by a variety of bacteria and some archaea. | |||
Sulfur is also important to those bacteria that use it during photosynthesis. Both green and purple sulfur bacteria can use sulfide and oxidize it to elemental sulfur during their photosynthesis reactions (Parkin, 1981). This being so, SRB and these green and purple sulfur bacteria can work in harmony with each other since each of their waste products can be part of the other one's energy source. This is not always the case, since the elemental sulfur that is released from these green and purple sulfur sometimes gets converted to sulfuric acid. Below are generic examples of both the purple and green sulfur and the SRB mediated chemical equations. | |||
<br><b>Purple and Green Sulfur Bacteria Photosynthesis:</b> H<sub>2</sub>S+CO<sub>2</sub>=CH<sub>2</sub>O+H<sub>2</sub>O+2S | |||
<br><b>SRB Respiration:</b> CH<sub>2</sub>O+S=CO<sub>2</sub>+H<sub>2</sub>S | <br><b>SRB Respiration:</b> CH<sub>2</sub>O+S=CO<sub>2</sub>+H<sub>2</sub>S | ||
====Methanogenesis==== | |||
Meromictic lakes are also a great habitat for methanogenesis to occur. Methanogenesis is a process that reduces carbon dioxide to methane. Carbon dioxide is the least efficient terminal electron acceptor in the electron tower. The process of methanogenesis is very unique, and only archaea can do it. Since methanogenesis is a very inefficent way to help produce energy, it will not be used unless every other electron acceptor is gone. Therefore, this process may be limited to deep within the monimolimnion. Archaea that can produce methane are called [[methanogens]]. | |||
==Current Research== | ==Current Research== | ||
Ryuji Kondo and research team looked at the sulfur reducing bacteria, SRB, in a meromictic lake, Lake Suigetsu, in Japan. In their study, they used PCR amplification with new assays to determine what sulfur reducing bacteria were present. The highest density of SRB occurred around the chemocline,which was six meters under the surface of the water. The largest populations of SRB included Desulfococcus multivorans | Ryuji Kondo and research team looked at the sulfur reducing bacteria, SRB, in a meromictic lake, Lake Suigetsu, in Japan. In their study, they used PCR amplification with new assays to determine what sulfur reducing bacteria were present. The highest density of SRB occurred around the chemocline,which was six meters under the surface of the water. The largest populations of SRB included [[Desulfococcus]] multivorans and [[Desulfonema limicola]]. These bacteria are all gamma-Proteobacteria. The Desulfobulbaceae family was the second greatest group of SRB. | ||
Lise Ovreas and research crew studied Lake Salenvannet, a meromictic lake in Norway in 1997. In this project, both bacteria and archaea populations were studied based on depth.The greatest amount of bacteria were found at the aerobic-anaerobic interface. The oxic zone had the greatest diversity, whereas the anoxic zone had three main bacterial populations. This is opposite of what was observed for the populations of archaea. Archaea seemed to have greater diversity in the anoxic zones. | |||
Lake Salenvannet was also studied with P. Tuomi and research crew. They found eight distinct bacteria biotypes. Most of the bacteria they found were at the 2.25 meter depth. This is consistent with the majority of bacteria found around the chemocline. Most of the bacteria that were found were | Lake Salenvannet was also studied with P. Tuomi and research crew. They found eight distinct bacteria biotypes. Most of the bacteria they found were at the 2.25 meter depth. This is consistent with the majority of bacteria found around the chemocline. Most of the bacteria that were found were determined to be copiotrophs that can rapidly increase their populations when excess nutrients were available. | ||
==References== | ==References== | ||
| Line 72: | Line 71: | ||
Friends of Ecological Reserves: http://ecoreserves.bc.ca/2012/02/11/images-of-amoebobacter-and-copepods-in-mahoney-lake/ | Friends of Ecological Reserves: http://ecoreserves.bc.ca/2012/02/11/images-of-amoebobacter-and-copepods-in-mahoney-lake/ | ||
Horne A.J., and C.R. Goldman 1994. <i>Limnology. 2nd edition.</i> New York, N.Y. McGraw-Hill, Inc. | |||
Kennesaw State University: http://science.kennesaw.edu/~jdirnber/BioOceanography/Lectures/LecPhysicalOcean/LecPhysicalOcean.html | Kennesaw State University: http://science.kennesaw.edu/~jdirnber/BioOceanography/Lectures/LecPhysicalOcean/LecPhysicalOcean.html | ||
| Line 82: | Line 83: | ||
Pfennig, N. 1977. "Phototrophic green and purple bacteria: a comparative systematic survey". Ann. Rev. Microbiol. 31:275-290. | Pfennig, N. 1977. "Phototrophic green and purple bacteria: a comparative systematic survey". Ann. Rev. Microbiol. 31:275-290. | ||
Sondergaard M. 2009. <i>Redox Potential. Vol. 2,</i> pp.852-859. In: G.E. Likens Encyclopedia of Inland Waters. Oxford: Elsevier | |||
Stewart K.M., K.F. Walker, and G.E. Likens 2009. <i>Meromictic Lakes. Vol. 2,</i> pp.589-602. In: G.E. Likens Encyclopedia of Inland Waters. Oxford: Elsevier | |||
The Encyclopedia of Earth: http://www.eoearth.org/article/Chemical_properties_of_lakes | The Encyclopedia of Earth: http://www.eoearth.org/article/Chemical_properties_of_lakes | ||
| Line 93: | Line 98: | ||
Water Quality Assessments-A Guide to Use of Biota, Sediments and Water in Environmental Monitoring-Second Edition: http://www.who.int/water_sanitation_health/resourcesquality/wqachapter7.pdf | Water Quality Assessments-A Guide to Use of Biota, Sediments and Water in Environmental Monitoring-Second Edition: http://www.who.int/water_sanitation_health/resourcesquality/wqachapter7.pdf | ||
Wikipedia | Wikipedia: http://en.wikipedia.org/wiki/Black_Sea | ||
Wikipedia: http://en.wikipedia.org/wiki/Sulfur-reducing_bacteria | |||
Yannarell A.C., and A.D. Kent 2009. <i>Bacteria Distribution and Community Structure.,</i> pp.201-210. In: G.E. Likens Encyclopedia of Inland Waters. Oxford: Elsevier | |||
Edited by | Edited by Tyler Groh, a student of Angela Kent at the University of Illinois at Urbana-Champaign. | ||
<!-- Do not edit or remove this line -->[[Category:Pages edited by students of Angela Kent at the University of Illinois at Urbana-Champaign]] | <!-- Do not edit or remove this line -->[[Category:Pages edited by students of Angela Kent at the University of Illinois at Urbana-Champaign]] | ||
Latest revision as of 00:26, 21 April 2012
Introduction
The Encyclopedia of Inland Waters defines meromictic lakes as stratified lakes that consists of two layers that do not completely mix. The top layer, the mixolimnion, is a portion of the lake, usually ending a couple of meters under the surface of the lake, that is exposed to the atmosphere. The mixing of the mixolimnion usually occurs for the majority of the year, especially in the northern latitudes where the mixolimnion does not stratify based on temperature. The one part of the year where these northern meromictic lakes' mixolimnions do not mix is during the winter when ice is covering the surface of the lake. The southern meromictic lakes may have stratified mixolimnions based on temperature and ultimately water density. These mixolimnions may not fully mix during the summer, but they will mix completely from late fall through early spring. The bottom layer, the monimolimnion, is cut off from the atmosphere by the mixolimnion. These two layers are separated by either a thermocline or a chemocline (Water Quality Assessments). The Encyclopedia of Inland Lakes further discribes this separation. A thermocline exists when there is a temperature difference between the surface and bottom of a lake. This temperature difference causes a difference in water density with the heavier cold water near the sediments. This temperature difference holds as long as the temperature of the lake does not cool off. Therefore, meromictic lakes that are stratified by a thermocline are typically found near the tropics where the surface of lakes never freeze. This is not to say that a thermocline cannot separate the mixolimnion and the monimolimnion in temperate climates. However, if this is the case, the lake needs to have a small surface area compared to its depth. This also means that the sides of the lake need to be steeply sloped. The small surface area compared to a lake's depth helps prevent the wind from being able to fully mix the lake when the temerature of the lake is uniform from late fall through early spring. After all, the larger the fetch, the length of a lake exposed to a gust of wind, is , the more likely the lake will turn completely over. This is especially true for shallow lakes with large fetches. The Encyclopedia of Inland Lakes gives a second way for lakes to be stratified into permanently different layers: the presence of a chemocline. A chemocline, in the context of meromictic lakes, is a gradient of salinity. The saltier, and more dense, water would settle to the bottom of the lake therefore forming the monimolimnion. The mixolimnion would have water with significantly less salt dissolved in it. These lakes are somewhat common in deep basins near coastal areas where fresh and salt water interact with each other.
The size of meromictic lakes vary greatly with the largest being the Black Sea. The Black Sea is about 436,400 square kilometers and has a maximum depth of 2,212 meters. This great depth and the fact that the bottom water is more saline than the top is what keeps the Black Sea from fully mixing. Regardless of its size,a meromictic lake is home to a very diverse set of species, especially when it comes to microbial communities. Although individual species do vary depending on which lake is being studied, multiple studies have indicated that there are a few aerobic species at the top of the mixolimnion. Populations and the diversity of the bacteria tend to increase as the thermocline or the chemocline is reached just a couple of meters under the surface of the lake (Ovreas, 1997). This barrier between the two pools of water seems to be the place that has the most amount of microbial species and therefore the highest biodiversity. After all, both aerobes and anaerobes can potentially be within a few meters of each other. Also, like the mixolimnion, light can reach this area, so both photoautotrophs and heterotrophs can potentially exist in this region. Past the chemocline or thermocline is the monimolimnion which is depleted of oxygen. Therefore, only anaerobic bacteria and archaea can function in this zone. Since the monimolimnion hardly ever, if at all, mixes, it is a very anaerobic place where sulfur redox reactions dominate. Methanogenesis, via methanogens, also occurs in the monimolimnion.
The Environment
The Physical Environment
Temperature
Since most meromictic lakes are deep and have a thermocline, temperature tends to be important. Temperature varies in the different layers of a meromictic lake. The surface of a meromictic lake is the warmest in the summer (Stewart, 2009). In fact, the mixolimnion may be stratified as in the picture to the left. In this depiction, there is an epilimnion, a metalimnion, and a hypolimnion in the mixolimnion. This stratification is caused by varying temperatures with the warmest water being in the epilimnion and the coldest in the hypolimnion. This separation will affect the way the lake mixes during the summer, and more than likely only the epilimnion and the top of the metalimnion will be able to mix well. However, once the colder fall temperatures arrive, this stratification ends up breaking apart, and the whole mixolimnion is allowed to mix entirely again. Regardless, the monimolimnion always remains separate from the mixolimnion, and therefore is not ever in contact with the atmosphere (Stewart, 2009).
Density
Density is another important physical property in meromictic lakes. Temperature plays a key role in determining the density of the water in the lake. This is why the epilimnion, having the warmer, less dense water, is the top layer of the mixolimnion, and why the hypolimnion, containing the colder, more dense water, is the bottom layer of the mixolimnion during the summer months. Another important factor that affects the density of water is the amount of solutes dissolved in the water, or in this case, the salinity (Stewart, 2009). The more saline the water, the more dense it is. Therefore, very saline water will stay right above the sediments of meromictic lakes. The gradient in salinity is the reason for the chemocline, and is why the hypolimnion in the mixolimnion is not a part of the monimolimnion even though they are roughly the same temperature. This varying in density throughout the meromictic lakes, especially in summer, is what controls most of the chemical processes within the lake.
The Chemical Environment
Dissolved Oxygen
Dissolved oxygen is the amount of oxygen that is held, dissolved, in water. The amount of oxygen that is dissolved in the water is greatest in the epilimnion of the mixolimnion and is lowest in the monimolimnion (Horne,1994). The epilimnion has the greatest amount of contact with the atmosphere and can be thoroughly mixed. This makes it very easy for the oxygen in the atmosphere to dissolve into the epilimnion. Some of this oxygen is able to get mixed in with the top of the metalimnion, but nothing beyond that. Also, since the epilimnion has a warmer temperature than the layers below it, it can hold less oxygen. However, the continuous mixing of the epilimnion during the summer keeps this layer oxygenated. Another way that a lake is able to obtain oxygen is through the photosythesis of plants and phototrophic microbes. Since plants and some microbes need light to survive, they can are limited to the top layers of the lake. These top layers are referred to as the photic zone, and is the area where photosynthesis takes place (Kennesaw State University). The lower layer of the photic zone is where 99 percent of the light from the sun is absorbed by the water. Not all organisms in water contribute to the dissolved oxygen concentration. Some, like fish, heterotrophic bacteria, decomposers, and benthic invertebrates, deplete the oxygen concentration. However, the epilimnion is usually robust enough to support these oxygen utilizing organisms.
The metalimnion is the portion of the meromictic lake that starts to show an oxygen deficiency, especially the lower portion of the metalimnion (The Encyclopedia of Earth). Even though the metalimnion has a cooler temperature than the epilimnion, and can therefore hold more oxygen, it will have a lower dissolved oxygen concentration due to the fact that it is not in direct contact with the atmosphere and cannot be mixed. To add to this lack of oxygen, the aerobic microbes and fish that live in this region of the lake will eventually deplete as much oxygen as they can before they have to move to a different location or die. This is even more true for the hypolimnion and the monimolimnion. In fact, since the monimolimnion rarely, if ever, gets mixed and it does not have organisms that photosynthsize, it usually only has a dissolved oxygen concentration of less than 1 ppm (CWTA). This is far below the amount of oxygen that most fish need to survive.
Redox Gradient
Lakes that are stratified, such as meromictic lakes, tend to have a redox gradient between the epilimnion and the hypolimnion, or in the case of meromictic lakes, between the mixolimnion and the monimolimnion (Sondergaard, 2009). This is especially true in eutrophic lakes due to the rapid depletion of oxygen in the summer due to decomposition. The presence of oxygen in the mixolimnion keeps the redox potential higher, and therefore, oxygen is used as a terminal electron acceptor for microbes. However, the deeper water in meromictic lakes has a lower concentration of dissolved oxygen, and therefore a lower redox potential. For instance, water with a dissolved oxygen concentration of 0.1mg/L has a redox potential of 200mV (Sondergaard, 2009). Once the oxygen is used up in an area that does not get readily supplied with oxygen, starting at the thermocline or chemocline, microorganisms have to switch electron acceptors. The next most energy favorable terminal electron acceptors include, but are not limited to, nitrate, manganese, iron, sulfate, and carbon dioxide. These are listed in order of most to least energetically favorable.
This redox gradient also occurs in the sediments of meromictic lakes (Sondergaard, 2009). If oxygen or nitrate is present in the sediments, they are usually in the topmost layer, which tends to be a brown color. A green layer is directly below this followed by a black horizon formed by iron and sulfate reduction respectively. The bottom most layer is usually a grey layer where methanogens are actively reducing carbon dioxide to methane. Redox gradients may also be seen in flooded soils.
Microbial communities
The Mixolimnion
The mixolimnion, since it can act like an individual lake and stratify, and since it has relatively low salinity in terms of the chemocline, can be home for numerous microbial communities that are present in freshwater lakes. These include, but are not limited to algae, actinobacteria, betaproteobacteria, alphaproteobacteria, gammaproteobacteria, cyanobacteria,and various types of freshwater, aerobic archaea (Yannarell, 2009). These microbial communities have adapted to use oxygen as their terminal electron acceptor in order to make as much energy, ATP, as they can. They also selected for living in open water with very little in terms of cover from predators. This means that they had to adapt in order to survive a predator's attack. Some of these adaptations include flagella, binding to larger particles, selecting for a larger or smaller cell body size, and working together as a community like algae do in algal mats.
The Chemocline
This zone of rapid change in salinity is in most meromictic lakes. However, there are a few rare meromictic lakes that do not have this chemocline and thus lack the microbial diversity of this section. The chemocline has the potential for the greatest amount of species diversity (Ovreas, 1997). Both fresh and saltwater microorganisms can survive within feet of each other in this layer, and so can aerobic and anaerobic microbes. The chemocline is also the area where the green and purple sulfur bacteria can survive. These bacteria need sulfur and light in order to conduct photosynthesis. This is not to say that these sulfur bacteria cannot survive in the mixolimnion, but rather, they have a greater population near the chemocline. The major adaptation for the microbes that exists in and near the chemocline is that they have to be metabolically diverse. In other words, facultative aerobes and anaerobes have the greatest chance of surviving near the chemocline. This is because oxygen concentrations have a tendency to fluctuate, especially during the summer months when the mixolimnion is stratified.
The Monimolimnion
In the deepest part of meromictic lakes, anaerobic bacteria and archaea dominate. There is little to no oxygen, therefore microbes will have to find the next best available terminal electron acceptor. SRB,some green and purple sulfur bacteria, and methanogens rule this portion of the lake. The main adaptation to living in this portion of the lake is that the microbe needs to be able to use sulfur or carbon dioxide in redox reactions in order to sustain their energy need.
Microbial processes
The Mixolimnion
The two major microbial mediated reactions that occur in the mixolimnion are photosythesis and aerobic respiration. Photosynthesis can be carried out by algae, cyanobacteria, and a few other microbes. This chemical process is essential to all life, and is a main source of carbon substrate for the life in a meromictic lake. When the organisms that fix carbon through photosynthesis die, they are either decomposed in the mixolimnion, or settle to the monimolimnion. Either way, organisms in both parts of the meromictic lake depend on this biomass for a media to live off of. The carbon dioxide taken in by these photosynthesis processes may come from respiration. Every organism respires to some extent. Aerobic respiration is an affective way in obtaining energy for the cell. General versions of photosynthesis and respiration are given below. Oxygen is used in the decomposition of the organic molecule in respiration since the mixolimnion, especially its epilimnion, has enough dissolved oxygen in order for aerobic decomposition to take place.
Photosynthesis: 6CO2+6H2O+light=C6H12O6+6O2
Respiration: C6H12O6+6O2=6CO2+6H2O
The Monimolimnion
Sulfur
Sulfur is a critical element starting at the chemocline and extending down to the monimolimnion. Since there is very little, if any, oxygen in these meromictic layers, the bacteria must find another terminal electron acceptor. Sulfur reducing bacteria, SRB, reduce elemental sulfur to sulfide (Kondo,2006). The other side to this redox reaction is that they oxidize organic compounds like acetate and succinate to carbon dioxide. This sulfur redox reaction can be done by a variety of bacteria and some archaea.
Sulfur is also important to those bacteria that use it during photosynthesis. Both green and purple sulfur bacteria can use sulfide and oxidize it to elemental sulfur during their photosynthesis reactions (Parkin, 1981). This being so, SRB and these green and purple sulfur bacteria can work in harmony with each other since each of their waste products can be part of the other one's energy source. This is not always the case, since the elemental sulfur that is released from these green and purple sulfur sometimes gets converted to sulfuric acid. Below are generic examples of both the purple and green sulfur and the SRB mediated chemical equations.
Purple and Green Sulfur Bacteria Photosynthesis: H2S+CO2=CH2O+H2O+2S
SRB Respiration: CH2O+S=CO2+H2S
Methanogenesis
Meromictic lakes are also a great habitat for methanogenesis to occur. Methanogenesis is a process that reduces carbon dioxide to methane. Carbon dioxide is the least efficient terminal electron acceptor in the electron tower. The process of methanogenesis is very unique, and only archaea can do it. Since methanogenesis is a very inefficent way to help produce energy, it will not be used unless every other electron acceptor is gone. Therefore, this process may be limited to deep within the monimolimnion. Archaea that can produce methane are called methanogens.
Current Research
Ryuji Kondo and research team looked at the sulfur reducing bacteria, SRB, in a meromictic lake, Lake Suigetsu, in Japan. In their study, they used PCR amplification with new assays to determine what sulfur reducing bacteria were present. The highest density of SRB occurred around the chemocline,which was six meters under the surface of the water. The largest populations of SRB included Desulfococcus multivorans and Desulfonema limicola. These bacteria are all gamma-Proteobacteria. The Desulfobulbaceae family was the second greatest group of SRB.
Lise Ovreas and research crew studied Lake Salenvannet, a meromictic lake in Norway in 1997. In this project, both bacteria and archaea populations were studied based on depth.The greatest amount of bacteria were found at the aerobic-anaerobic interface. The oxic zone had the greatest diversity, whereas the anoxic zone had three main bacterial populations. This is opposite of what was observed for the populations of archaea. Archaea seemed to have greater diversity in the anoxic zones.
Lake Salenvannet was also studied with P. Tuomi and research crew. They found eight distinct bacteria biotypes. Most of the bacteria they found were at the 2.25 meter depth. This is consistent with the majority of bacteria found around the chemocline. Most of the bacteria that were found were determined to be copiotrophs that can rapidly increase their populations when excess nutrients were available.
References
Connecticut Water Trails Association, CWTA: http://connecticutwatertrails.com/CWTA%20-%20Lake%20-%20Different%20Types%20Of%20Lakes%20-%20Meromictic%20Lake.htm
Friends of Ecological Reserves: http://ecoreserves.bc.ca/2012/02/11/images-of-amoebobacter-and-copepods-in-mahoney-lake/
Horne A.J., and C.R. Goldman 1994. Limnology. 2nd edition. New York, N.Y. McGraw-Hill, Inc.
Kennesaw State University: http://science.kennesaw.edu/~jdirnber/BioOceanography/Lectures/LecPhysicalOcean/LecPhysicalOcean.html
Kondo, R., Osawa, K., Mochizuki, L., Fujioka, Y., and Butani, J. 2006. "Abundance and diversity of sulphate-reducing bacterioplankton in Lake Suigetsu, a meromictic lake in Fukui, Japan". Plankton Benthos Res. 4:165-177.
Ovreas, L., Forney, L., Daae, F.L., and Torsvik, V. 1997. "Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA". Applied and Environmental Microbiology. 63:3367-3373.
Parkin, T.B., and Brock, T.D. 1981. "The Role of Phototrophic Bacteria in the Sulfur Cycle of a Meromictic Lake". Limnology and Oceanography. 26:880-890.
Pfennig, N. 1977. "Phototrophic green and purple bacteria: a comparative systematic survey". Ann. Rev. Microbiol. 31:275-290.
Sondergaard M. 2009. Redox Potential. Vol. 2, pp.852-859. In: G.E. Likens Encyclopedia of Inland Waters. Oxford: Elsevier
Stewart K.M., K.F. Walker, and G.E. Likens 2009. Meromictic Lakes. Vol. 2, pp.589-602. In: G.E. Likens Encyclopedia of Inland Waters. Oxford: Elsevier
The Encyclopedia of Earth: http://www.eoearth.org/article/Chemical_properties_of_lakes
Tuomi, P., Torsvik, T., Heldal, M., and Bratbak, G. 1997. "Bacterial Population Dynamics in a Meromictic Lake". Applied and Environmental Microbiology. 63:2181-2188.
University of Colorado:http://www.colorado.edu/eeb/EEBprojects/schmidtlab/studentres/EBIO3400/Lecture07.pdf
Water on the Web: http://www.waterontheweb.org/under/lakeecology/05_stratification.html
Water Quality Assessments-A Guide to Use of Biota, Sediments and Water in Environmental Monitoring-Second Edition: http://www.who.int/water_sanitation_health/resourcesquality/wqachapter7.pdf
Wikipedia: http://en.wikipedia.org/wiki/Black_Sea
Wikipedia: http://en.wikipedia.org/wiki/Sulfur-reducing_bacteria
Yannarell A.C., and A.D. Kent 2009. Bacteria Distribution and Community Structure., pp.201-210. In: G.E. Likens Encyclopedia of Inland Waters. Oxford: Elsevier
Edited by Tyler Groh, a student of Angela Kent at the University of Illinois at Urbana-Champaign.