Metabolism in Neisseria meningitidis
Introduction
By Molly Folks
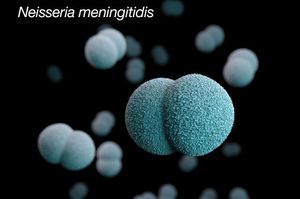
Neisseria meningitidis (N. meningitidis) is Gram-negative bacterium that is a member of the class, Betaproteobacteria. Neisseria are aerobic heterotrophic cocci, and the cocci of Neisseria genus form diplococci, distinctive pairs of cocci (Fig 1) [2]. Most members of the Neisseria genus are commensal organisms that colonize the nasal and oral mucosa of animals. There are 11 species of Neisseria that colonize humans and only 2 are pathogens, Neiserria meningitidis and Neiserria gonorrhoeae.
N. meningitidis was first discovered in 1887 by Anton Weichselbaum after analyzing the cerebrospinal fluid (CSF) of a patient who was infected with meningitis [3]. There are at least 12 known serotypes of N.meningitidis based on the unique capsular polysaccharides seen on the cell surface [3]. Serotypes are groups that are found within a single microorganism, such as viruses and bacteria, that share differentiating surface structures [4]. Serotypes A, B, C, W, X, and Y are the serotypes that cause most of the meningococcal infections [3]. Each of these common serotypes can be linked to various regions around the globe. The main serotypes causing meningococcal disease in Africa are serotypes A and C [3]. Serotypes B and C are the main serotypes seen in Europe and the Americas [3]. Serotype Y is what generally causes infection in the United States and Canada [3]. Epidemic outbreaks around the world are linked to serotype W [3].
N. meningitidis is one of the common and important causes of community-acquired bacterial meningitis in the United States [3]. Children and adults can be infected by this bacterium. The infection has a high mortality rate if not recognized and treated immediately following the diagnosis. N. meningitidis can lead to other infections as well such as; meningococcal septicemia, pneumonia, septic arthritis, pericarditis, and urethritis [3]. This bacterium can cause both endemic and epidemic infections, also having the ability to infect young, healthy adults [3].
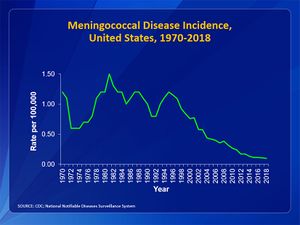
Due to the routine use of the meningococcal vaccination, the number of reported cases of meningococcal disease has decreased (Fig 1). In the United States, the number of cases each year is less than 1 case per 100000 [3]. February through March present the highest infection rates seen in the United States [3]. Infants less than 1-year old make up the highest rate of meningococcal disease seen with 5.38 cases per 100000 [3]. In sub-Saharan Africa, N.meningitidis has led to endemics and epidemic outbreaks and is an important cause of bacterial meningitis [3]. The mortality rate is very dependent on whether those who are infected have sought treatment or not. The mortality rate for individuals who have received treatment ranges from approximately 10 to 14% [3]. In patients who do not receive treatment, the mortality rate rises to 50% [3].
Invasive Behavior
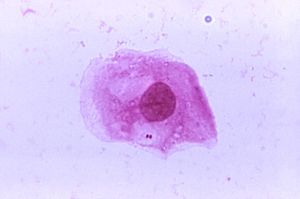
N. meningitidis or meningococci are obligate commensals in humans, meaning that the bacterium is colonizing the nasal and oral mucosa of humans without causing them harm. This phenomenon of the bacterium remaining in the mucosa without harming the human is known as carriage [6]. Approximately 10% of the population may carry N. meningitidis in the upper airway during nonepidemic situations and act as asymptomatic carriers [6]. In Fig 3, it can be seen that N. meningitidis can be found in locations other than the upper airway during carriage. The time in which one person remains in the carrier state can vary between being chronic, lasting for several months, intermittent or transient [6]. It has been seen that colonization of the meningococci can induce an antibody response from the three major immunoglobulin classes and may act as an immunizing event [6]. This situation will occur within a few weeks of obtaining the bacterium.
However, the bacterium does have the ability to enter the bloodstream. On occasion, shortly after colonization in the upper airway, N. meningitidis strains can penetrate the mucosal membrane and from there enter the bloodstream [6]. Once the bacterium has entered the bloodstream, various forms of disease caused by the bacterium may develop. It is important to note that non-carriers are at high-risk for meningococcal disease because it is unknown about their capability to maintain a commensal relationship with an acquired strain of N. meningitidis [6].
Risk Factors
As seen in most diseases, certain risk factors put some people at an increased risk of acquiring a meningococcal disease. These risk factors include age, group settings, certain medical conditions, and travel [8].
Age
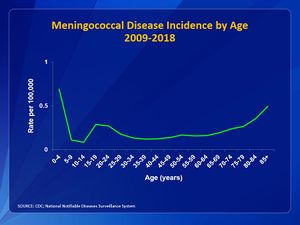
In the United States, infants, teens, and young adults have the highest incidence of meningococcal disease [7]. In Fig 4, the incidence of meningococcal disease among age groups is shown. It can be that the two largest peaks are at children less than 1-year-old and in teen and young adults from ages 16 to 23. Incidence drops after age 23. However, it can be seen that the rate begins to rise again after age 55, showing that people of old age are also at risk (Fig 4).
Group Settings
Wherever large groups are present, infectious diseases tend to spread. Recent data has shown that college students are at a higher risk for meningococcal disease than teens and young adults that are not attending college [9]. College campuses are the most known group settings for the spread of meningococcal disease, however, group settings can also include military recruits and even the common household [6].
Certain Medical Conditions
Some certain medical conditions and medications may weaken the immune system and increase the risk of a person acquiring a meningococcal disease. The CDC has made a recommendation that individuals with a persistent complement component deficiency need to receive 2 vaccines (a meningococcal conjugate (MenACWY) vaccine and a serogroup B meningococcal (MenB) vaccine) for protection against meningococcal disease [10]. A persistent complement component deficiencies are disorders affecting the complement system, which is what helps the body fight infections [10]. Other situations where an individual will require 2 vaccines are people who are taking complement inhibitors, people with functional or anatomic asplenia (a nonfunctional or missing spleen), and people with HIV [10].
Travel
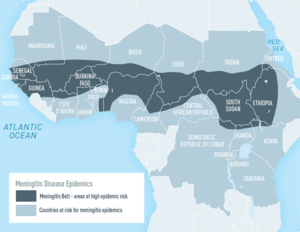
The meningitis belt is an area in sub-Saharan Africa that has a high rate of meningococcal disease. In this area, there are much higher rates of meningococcal disease than in the United States and outbreaks are very common [12]. In Fig 5, a map of Africa shows where the meningitis belt is located as well as surrounding areas that are also at risk for an outbreak. Because of this, people who live in or are traveling to the meningitis belt are recommended to receive the meningococcal conjugate vaccine[12]. The disease is most common in the meningitis belt during the dry season, which occurs from December through June [12]. Travelers who are spending a lot of time with local populations, especially during outbreaks, are at the highest risk of getting sick [12]. Even if a traveler has been vaccinated, if they continue to be at an increased risk, the traveler should receive booster doses of the vaccine [12].
Transmission
The bacterium is spread from one person to another through the sharing of respiratory and throat secretions (saliva and spit) [13]. Normally, it takes close, such as coughing or kissing, or lengthy contact to spread the bacterium [13]. It is important to note that N. meningitidis is not as contagious as germs that cause the flu or the common cold [13]. People are not able to acquire the bacterium through casual contacts, such as touching, or by breathing the air of where someone with meningococcal disease has been [13].
The bacterium does have the ability to spread to people that have been in close or lengthy contact with an individual with meningococcal disease. These people are now at an increased risk for infections and can include [13]:
-People in the same household
-Roommates
-Anyone that has been in direct contact with oral secretions, such as a boyfriend or girlfriend
Any person who has been in close contact with meningococcal disease should receive antibiotics to prevent themselves from getting sick. This is known as prophylaxis. This measure is not taken because the individual has the disease but rather to prevent the disease. Each case of meningococcal disease is investigated so every close contact and be identified and receive prophylaxis [13]. If an individual has not been in close contact with meningococcal disease, there is no need for that individual to receive prophylaxis [13].
Signs and Symptoms
The two most common meningococcal infections caused by Neisseria meningitidis are meningococcal meningitis and meningococcal septicemia or meningococcemia. Symptoms of meningococcal disease will appear as flu-like symptoms before becoming much worse and life-threatening [14]. Theses infections can become deadly in a matter of hours [14].
Meningococcal Meningitis
Meningitis that is caused by the bacterium N. meningitidis is known as meningococcal menigitis. When an individual has meningococcal meningitis, the bacterium has infected the lining of the brain and spinal cord resulting in swelling[14]. Below are symptoms of meningococcal meningitis[14]:
-Fever
-Headache
-Stiff neck
Some additional symptoms include[14]:
-Nausea
-Vomiting
-Photophobia (eyes being more sensitive to light)
-Altered mental status (confusion)
In newborns and babies, it may be difficult to recognize the classic symptoms from above [14]. Instead, it is important to take note of the following: inactivity, irritability, vomiting, feeding poorly, or the presence of a bulging in the soft spot of the skull (anterior fontanelle)[14]. Doctors may also look at a young child's reflexes for signs of meningitis[14].
Meningococcal Septicemia
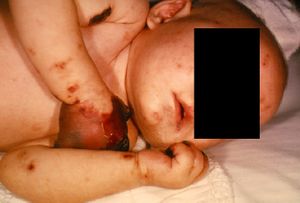
Septicemia (a bloodstream infection) caused by N. meningitidis is known as meningococcal septicemia or meningococcemia. When an individual has meningococcemia, the bacterium has entered the bloodstream and multiplied, thus damaging the walls of the blood vessels [14]. This leads to bleeding into the skin and organs [14]. Symptoms of meningococcemia include [14]:
-Fever and chills
-Fatigue
-Vomiting
-Cold hands and feet
-Sever aches or pain in the muscles, joints, chest, or abdomen
-Rapid breathing
-Diarrhea
-In the later stages, a dark purple rash (Fig 6)
It is crucial that if you recognize any of the symptoms listed above, for either meningococcal meningitis or meningococcemia, that you call the doctor right away.
Diagnosis and Treatment
Meningococcal disease is very serious and can become lethal to those infected in a matter of hours. It is crucial to have an early diagnosis and begin treatment as soon as possible.
Diagnosis
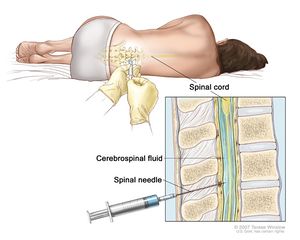
It is difficult to diagnose meningococcal disease because the disease exhibits signs and symptoms similar to those of other infections[15]. If it is suspected that a patient has a meningococcal disease, the doctor will collect samples of the patient’s blood or cerebrospinal fluid (fluid near the spinal cord)[15]. Fig 7 displays the procedure of a doctor collecting cerebrospinal fluid for diagnosis. The collected samples are then sent to a laboratory for testing. If N. meningitidis bacteria are found in the samples, the laboratory can make a culture of the bacteria[15]. By making a culture of the bacteria, the doctor will be able to know which specific type of N. meningitidis is infecting the patient[15]. Once the doctor knows what the patient is infected with, they can decide which antibiotic will work best[15]. Other tests can be used to detect and identify the type of bacteria if the cultures are not able to[15].
Treatment
To treat meningococcal disease, doctors will use a variety of antibiotics. Treatment of meningococcal disease should begin as soon as possible. If a doctor suspects that a patient may be infected with meningococcal disease, the doctor will begin to give the patient antibiotics immediately[15]. The administering of antibiotics is to reduce the chances of the patient dying due to the infection[15]. Depending on the severity of the infection, the doctor may need to use further treatment methods such as[15]:
-Breathing support
-Medications to treat low blood pressure
-Surgery to remove dead tissue
-Wound care for parts of the body with damaged skin
Complications
There is the possibility to experience complications during treatment. 10 to 15 in 100 people who are infected with meningococcal disease and have received antibiotic treatment will die[15]. Of those who survive the infection, up to 1 in 5 survivors will suffer from long-term impairments, such as hearing loss, nervous system problems, brain damage, or loss of limb(s)[15].
Prevention
Many steps can be taken to prevent meningococcal disease. The best method of prevention for meningococcal disease is keeping up to date with the recommended vaccines[16]. Other practices, such as getting enough rest and staying away from other people who are sick, also work well in preventing meningococcal disease[16].
Vaccination
There are vaccines that will help protect again the three serotypes (B, C, and Y) of N. meningitidis most commonly seen in the United States[16]. However, individuals are still at risk for developing meningococcal disease after vaccination as like with any vaccine, the meningococcal vaccines are not 100% effective[16].
Antibiotics
Prophylaxis involving antibiotics should only be used after being in close contact with someone who has meningococcal disease. This is to prevent the individual from getting sick from the bacteria they may now be hosting in their body.
Re-Infection
There is the rare possibility that an individual may become infected with meningococcal disease after previously having it[16]. Previous infection does not guarantee future protection from infections[16]. Because of this, the CDC recommends that all preteens and teens receive meningococcal vaccines[16]. There are also times when children and adults should get vaccinated for meningococcal disease[16].
Metabolism and Virulence
It is known N. meningitidis can penetrate the mucosal membrane and enter the bloodstream, leading to the development of meningococcal disease in the host. The question surrounding N. meninigitidis is what causes the bacteria to have this invasive behavior. It has been discovered that invasive behavior seen is not a part of the normal meningococcal life cycle because once the bacteria have entered the bloodstream or the central nervous system, they cannot be transmitted to other hosts[17]. In attempts to find genes that code for genuine virulence factors, such as a polysaccharide capsule, and that are common to and at the same time tethered lone to hyperinvasive strains of N. meningitidis have failed [17]. Many of the “virulence genes” have been found in neisserial species that are strictly commensal. This lack of complete understanding of meningococcal virulence challenges many concepts present in infection biology.
Recent studies have determined that the ability of a pathogen to successfully adapt to and survive within the environment in which it is located in terms of nutrient assimilation is very important for pathogenesis[17]. An example of this is that many pathogens have to steal amino acids from their hosts to produce proteins. The bacteria have then developed new, varied means to undermine the mechanism that has been developed by the host to starve the bacteria from these required nutrients [17]. “Nutritional virulence” is a term to describe the mechanisms that target the major host biosynthetic pathways or nutrient-rich sources to improve the host supply of limited resources[17]. Bacteria need these resources to metabolize and grow within the host. It is known that the host environments in which meningococci colonize pose challenges. These challenges occur in terms of nutrient availability and host immune effectors[17]. Because these challenges are known, the core metabolism of the N. meningitidis in the context of colonization and invasion of the host tissues.
The metabolism of the meningococci in the carrier state and the invasive state must be analyzed to fully understand the effect of metabolism on virulence. N. meningitidis is typically known as aerobic heterotroph. However, it has been discovered that meningococci also can denitrify nitrite (it cannot denitrify nitrate). This process of denitrifying nitrite can support the growth of meningococci[18] . The N.meningitidis genome only possesses a few genes that are likely to be involved in denitrification[18]. Yet, because these genes are expressed in both the carrier state genome and in the invasive state genome, it is difficult to connect denitrification to virulence other than granting the bacteria the ability to metabolize anaerobically.
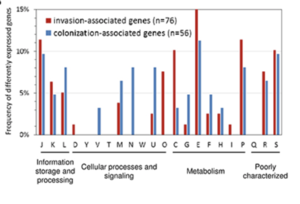
After analysis of the genomes from colonization-associated and invasion-associated bacteria, it was seen that the metabolic profile was different between the two groups of genes [17]. There were also differences in the functional profile of genes from colonization-associated bacteria and invasion-associated bacteria. In Fig 8, the differences in the frequency of different gene expression can be seen as the association of the bacteria changes from colonization to invasion. The differences that were seen may be linked to the new environments or goals of the bacteria. During colonization, the bacteria are attached to airway epithelial cells. After the bacteria have invaded the bloodstream, the bacteria are in a completely different environment. The bacteria have to attach to different cells and are looking to infect the host now rather than just live on airway epithelial cells.
One experiment, performed in 2004, explored how knocking out the gdhA gene would affect the metabolism and virulence of meningococci. By knocking out the gene, they were able to see the importance of the glutamate dehydrogenase gdhA had on glutamate metabolism and virulence[19] . It was also found that gdhA was found to be essential for the survival of meningococcal survival in infant rat models[17].
Conclusion
Metabolic adaptation does play a major role in enabling the bacteria to exploit host resources to favor themselves in their interactions with the host. However, it was difficult to fully understand the importance of nutritional virulence due to inadequate animal models because N. meningitidis is so adapted to the human host[17]. If there was a better model system to mimic human conditions, then phenotypic differences between carrier strains and strains that come from hyperinvasive lineages[17]. This research about the connection between virulence and metabolism can help scientists and doctors better understand how N. meningitidis infects the human host and becomes pathogenic. By having a better understanding of how the bacteria infects the human host, it can further the understanding of how vaccines can prevent meningococcal infection and how antibiotics can kill off the bacteria once someone becomes infected with the disease. Could it be possible that an antibiotic can be produced to alter the metabolism of the bacteria to prevent the bacteria from becoming virulent? Further exploration of metabolism can further the understanding of N. meningitidis and other bacteria.
References
- ↑ 1.0 1.1 1.2 Meningococcal Photos, CDC, Accessed April 20th, 2020
- ↑ Slonczewski, J., and Foster J. W.. Microbiology: An Evolving Science. New York
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 [Nguyen N, Ashong D. Neisseria Meningitidis. [Updated 2020 Mar 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549849/ ]
- ↑ Serotypes and the Importance of Serotyping Salmonella, CDC, Accessed April 20th, 2020
- ↑ Surveillance, CDC, Accessed April 20th, 2020
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Yazdankhah, S. P., & Caugant, D. A. (2004). Neisseria meningitidis: an overview of the carriage state. Journal of Medical Microbiology, 53(9), 821–832. doi: 10.1099/jmm.0.45529-0
- ↑ 7.0 7.1 Age as a Risk Factor, CDC, Accessed April 20th, 2020
- ↑ Risk Factors, CDC, Accessed April 20th, 2020
- ↑ Group Settings as a Risk Factor, CDC, Accessed April 20th, 2020
- ↑ 10.0 10.1 10.2 Certain Medical Conditions as a Risk Factor, CDC, Accessed April 20th, 2020
- ↑ Travel-Related Infectious Diseases, CDC, Accessed April 20th, 2020
- ↑ 12.0 12.1 12.2 12.3 12.4 Travel as a Risk Factor, CDC, Accessed April 20th, 2020
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 Causes and Spread to Others, CDC, Accessed April 21st, 2020
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 Signs and Symptoms, CDC, Accessed April 21st, 2020
- ↑ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 15.11 Diagnosis, Treatment, and Complications, CDC, Accessed April 21st, 2020
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 Prevention, CDC, Accessed April 22nd, 2020
- ↑ 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 Schoen, C., Kischkies, L., Elias, J., & Ampattu, B. J. (2014). Metabolism and virulence in Neisseria meningitidis. Frontiers in Cellular and Infection Microbiology, 4. doi: 10.3389/fcimb.2014.00114
- ↑ 18.0 18.1 M. F., Stevanin Tânia M., Read, R. C., & Moir, J. W. B. (2002). Nitric Oxide Metabolism in Neisseria meningitidis. Journal of Bacteriology, 184(11), 2987–2993. doi: 10.1128/jb.184.11.2987-2993.2002
- ↑ C., Salvatore, P., Vitis, L. R. D., Colicchio, R., Monaco, C., Tredici, M., … Alifano, P. (2004). Regulation and differential expression of gdhA encoding NADP-specific glutamate dehydrogenase in Neisseria meningitidis clinical isolates. Molecular Microbiology, 51(6), 1757–1772. doi: 10.1111/j.1365-2958.2003.03947.x
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.
