Microbial Infection of Burn Wounds
By Erin Pienciak
Overview of Burns
Burns are damage to the skin caused by chemicals, electricity, heat, sunlight or nuclear radiation [8]. Overall burn severity is determined based on the degree of tissue damage and the size of the area affected. Approximately 500,000 burns are treated each year by hospitals across the United States; 40,000 required prolonged hospitalization [2]. Caucasian males are the most likely demographic to sustain a burn injury [2].
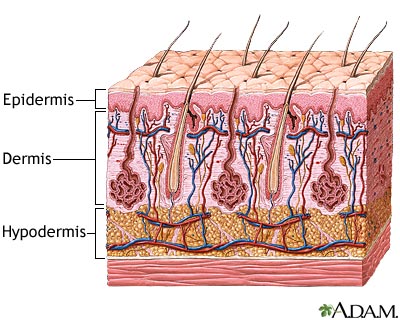
The tissue damage incurred is classified into three categories: first, second and third-degree burns [3]. First-degree burns involve only damage to the topmost layer of the skin, the epidermis. Second-degree burns contain damage to the epidermis as well the dermis, the underlying layer of the skin. Third-degree burns refer to damage or destruction of the entire depth of the skin as well as tissues that lie beneath it. These are three-dimensional injuries with damage extending in all direction from the center of the injury.
The area of a burn is often determined using the “Rule of Nines,” which divides the body up into sections that correspond with approximately 9% of the body’s surface area [3]. For example, an arm, the abdomen and the entire head each account for 9%. If all three of these body parts were burned, it would be estimated that the patient sustains injuries to 27% of his body. Burn severity is determined by combining information about the source of the burn, the type of damage done and the extent of the body injured. In addition, burn location is important. For example, burns to the face, hands or feet are considered severe because of the resulting complications with airway management, dexterity and movement.
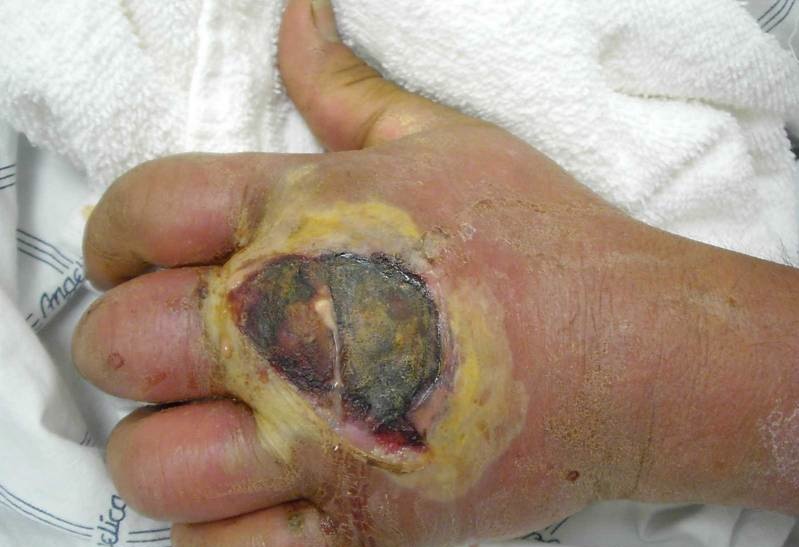
Burns wounds have three separate zones of concern. The zone of coagulation is located where the skin came in contact with the burn source, at the center of the wound. It is made up of dead, leathery tissue that forms the burn eschar, or scab. The zone of stasis surrounds the zone of coagulation; tissue is this zone is alive but at a high risk of infection and necrosis due to decreased perfusion to the area. Lastly, the zone of hyperemia surrounds the zone of stasis and contains healthy skin though vasodilatation in this area is common as a result of the injury [3]. Signs of infection include change in color of the wound, spontaneous separation of the eschar, cellulitis and graft loss [8]. These signs may be accompanied by localized redness, heat and tenderness [8]. Sepsis occurs when microbes or their toxins have expanded past just the wound to infect the rest of the body; this is characterized by high fevers, accelerated heart rate, increased respirations per minute and hyperglycemia [8].
Infection of burns is common because the skin, a physical barrier against microbes, has been compromised. Furthermore, in moderate and severe burns the underlying vasculature of the skin has been damaged or destroyed and so immunity agents, such as T cells, cannot reach sites of infection. Accordingly, the risk of infection increases proportionately with the size of the burn [3].
Burn Infections
Burn wound infection is problematic because it delays healing, encourages scarring and may result in bacteremia, sepsis or multiple-organ dysfunction syndrome (a.k.a. organ failure) whereby organs from several systems are unable to maintain homeostasis on their own, requiring immediate medical attention [3].
Bacteria and fungi are the most common pathogens of burn wounds. These microbes form multi-species biofilms on burn wounds within 48 – 72 hours of injury [3]. Organisms originate from the patient’s own skin, gut and respiratory flora, as well as from contact with contaminated health care environments and workers [3, 8]. Gram-positive bacteria are some of the first to colonize burns, followed quickly by gram-negative . Fungal infection tends to occur in the later stages after the majority of bacteria have been eliminated by topical antibiotics [3]. Two bacterial species, methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas areuginosas will be examined in depth in this page as they are two of the most prevalent infective agents. These two species have proven particularly difficult to treat because they posses a large number of virulence factors and antimicrobial resistance genes.
methicillin-resistant Staphylococcus aureus
MRSA are gram-positive, spherical microbes and some of the earliest colonizers of burn wounds. Thsee bacteria possess capabilities for both aerobic and anaerobic metabolism. Their genome is contained on one circular chromosome, with antibiotic resistance encoded in transposons. MRSA dwell in the sweat glands, hair follicles and mucous membranes of humans. MRSA can be difficult to eradicate because they often can colonize a host for a long time before causing an infection; until symptoms of infection emerge, MRSA remains undetected an untreated [13].
Staphylococcus aureus resistance to methicillin and other similar antibiotics is due to the altered structure of penicillin binding proteins. This mutation is caused by resistance genes that are carried in the staphylococcal cassette chromosome (SCC) mec, a mobile genetic element. The cassette encodes for an insertion sequence element, recombinases and regulatory genes. Five versions of SCCmec have been identified, each of which confers resistance to slightly different sets of agents [13].
Besides those that confer antibiotic resistance, MRSA virulence factors include adhesion proteins, colonization proteins, superantigens, exotoxins, and pore-forming toxins. Adhesion proteins allow MRSA to adhere exceptionally well to host tissues, which makes it difficult for the body to clear the pathogens. Colonization proteins help the bacteria to compete more effectively with other bacteria, both within their own species and with others. Some of these proteins also increase salt tolerance which could be beneficial in instances of infection where low perfusion results in high external osmolarity, such as in burn wounds. Superantigens instigate an elevated immune response in the body, sometimes up to 20% greater than would be triggered by bacteria not in possession of this virulence factor. This is particularly dangerous for patients because it can cause high fevers, shock, multiple organ failure and/or toxic shock syndrome. Pore-forming toxins can lead to vascular leakage, hypovolemic shock and tissue necrosis. It is for these reasons that MRSA infections are so dangerous to patients whom are already immuno-compromised [13].
Pseudomonas areuginosa
P. aeruginosa are the most common source of burn infections [3]. They are gram-negative bacilli that possess a single supercoiled circular chromosome. P. aeruginosa are facultative aerobes capable of fermentation that live in the human gut. These bacteria are known for their tendency to cause disease in immuno-compromised patients, such as those with AIDS or cystic fibrosis, but rarely in healthy individuals [4].
P. aeruginosa use quorum sensing to induce the production of virulence factors such as proteases, hemolysins, exotoxin A and pyocyanin [4, 11]. These bacteria posses two quorum-sensing systems: one that regulates proteases and another that regulates hemolysins. Though separate systems, the two interact through products of the protease system controlling the hemoysin system at both transcriptional and posttranslational levels [11]. While it has been widely held that quorum sensing is required for biofilm formation, new research has been put forth that challenges that position. A team of scientists out of Auburn University investigated the development of P. aeruginosa biofilms using a mouse burn model [10]. Third-degree thermal injury was induced and mice were then infected with two strains of P. aeruginosa, one wild type (WT) and the other quorum-sensing deficient (QS). Eschar samples were examined using flouresence and scanning electron microscopy at 8, 24 and 48 h after infection. Both strains developed biofilms at the same pace, although WT biofilms were somewhat denser than the QS strain. It had been previously demonstrated that the WT strain was more virulent than the QS. This study suggests that the difference in virulence is not due to impaired biofilms formation, rather it is likely due to differences in the expression of virulence factors. It seems that quorum-sensing knockout strains lost some other genes in the process of mutation.
Proteases are virulence factors that degrade the integrity of the host’s physical barriers by splitting proteins and amino acids, allowing for deeper infiltration of infection [4]. Exotoxin A halts the synthesis of proteins, causing local tissue damage, immunosuppression and cell death [11]. Hemolysins act like detergents to break down lipids in epithelial cells allowing, like the proteases, the bacilli to penetrate the host resulting in the spread of infection [11]. In addition, genes encoding proteins for pili and flagella are also important to P. aeruginosa since without these structures, bacteria are unable to navigate their environment and form biofilms [4]. P. aeruginosa infections are difficult to treat because these microbes possess multiple strategies for eluding predators, including multidrug efflux pumps, antibiotic-modifying enzymes, and tough outer membranes with low permeability [11].
Treatment
Burn patients have a uniqe set of requirements while recovering from their trauma. Of the utmost importance is airway, breathing and circulation maintenance. Patients may have sustained inhalation injuries when they were burned and thus could require additional interventions. Hemodynamic homeostasis can also be problematic as a result of disrupted blood flow and fluid loss. Furthermore, the metabolism of burn victims actually increases in response to the injury and so nutritional intake must increase accordingly [3]. Interestingly, meeting the nutritional needs of gastrointestinal flora can help mitigate wound infection because they do not need to go in search of a food source. All of these issues can affect temperature regulation, so care must be taken that a patient stays within normal limits. Pain management also needs to be considered. These issues must be addressed before the burn itself can be treated.
Traditional
For a long time, silver-containing compounds were used to eliminate burn wound infection and inflamation. These compounds are available in many forms, including creams, ointments and bandages. Originally, commercial dressings were simply saturated with the silver solution. Later models of these products used slow-releasing compounds so that bandages could be changed less frequently to minimize disturbance of the re-growing skin. Moreover, silver operates over a broad-spectrum, and is effective against both fungi and bacteria, including MRSA. Silver-resistance is a very rare phenomenon, which makes it an attractive alternative to antimicrobial pharmaceuticals. However, in the early twenty-first century it was realized that silver compounds actually slow the healing process and can be toxic to certain host cells. Silver’s antibiotic properties depend upon its ability to readily bind with negatively charged molecules. This binding though is fairly indiscriminant and so can attack healing host cells as well as microbial cells, especially in the presence of wound fluids. Delays in healing are specifically caused by retarded sloughing of necrotic tissues. The build-up of silver compounds in the blood and kidneys of burn patients has been observed, with some sparse reports of corresponding cytotoxic effects. Since the goal of burn treatment is to close the wound as quickly as possible, the use of silver-containing products has experienced a dip in popularity [1].
Currently, the most widely accepted methods of treatment of moderate and severe burns include wound excision and the application of topical antimicrobial agents [3]. The presence of the eschar tends to produce localized inflammation. The purpose of excision is to surgically remove the eschar, thereby reducing inflammation and minimizing scaring. The wound can then be debrided, cleansed and closed using a skin graft or a synthetic alternative [3].
In order to not encourage resistance, topical antimicrobials must be specific to the species colonizing a wound. Species can be discerned by taking a simple swab culture of the wound [3]. Oral antibiotics are not widely used because they have been shown to be largely ineffective in preventing infection, and may actually promote infection by encouraging colonization by antibiotic-resistant strains [3]. Laminar-airflow systems are another precaution taken against infection that is typically utilized in burn units [3]. In laminar flow systems, air only flows in one direction (out of the room through a vent), which reduces the chance of contaminated air circulating to other areas of a floor or building. One of the most effective ways to reduce the spread of antibiotic-resistant bacteria is simply for healthcare workers to use gloves whenever working with a burn patient or their environment, and to change into new gloves upon contact with a new patient [9].
Alternative and Developing
Researchers are looking to develop alternative therapies to commercial antibiotics due to the emergence of resistant strains. The use of essential oils to combat infection has intrigued the pharmaceutical industry, possibly due to growing enthusiasm for organics and all-natural products in other sectors such as food and make-up. Edward-Jones and colleagues (2004) investigated the usefulness of lavender, geranium, patchouli, and tea tree oils, as well as grapefruit seed extract, in treating MRSA infections. Essential oils were tested singly and in various combinations in vitro. Solutions were placed on filter paper disks and were either placed directly on the agar plates or secured to the top of the Petri dish so that the vapors alone had contact with bacteria. Direct contact with tea tree oil was shown to be the most effective against MRSA out of the individual oils, while the combination of geranium oil and grapefruit seed extract was especially successful against MRSA as a vapor. While these results are auspicious, essential oils can be highly toxic and irritating, warranting caution as the leap is made from in vitro to in vivo studies.
Honey is another ancient burn remedy that has piqued curiosity in the medical community as of late. The successful use of honey to treat an array of wounds, not just burn, has been observed in a wide array of venues including anecdotal evidence, animal models and random assignment in clinical trials. Honey acts to heal wounds via an array of mechanisms, resulting in very quick recovery times. It sloughs necrotic tissues and the eschar rendering debridement and excision unnecessary. In addition, honey stimulates reconstruction of the skin, blood vessels and connective tissues. It possesses both antibacterial and anti-inflammatory properties, and has even been shown to be effective in killing S. aeruginosa. Of no small importance, honey deodorizes putrefied wounds, making them more pleasant to deal with for all involved. In some instances these characteristics are so effective in healing the wound that skin grafting is not necessary. One study compared the speed of infected wound recovery using honey or silver sulfadiazine as treatment, with compelling results. After seven days, 91% of patients treated with honey had sterile wounds while only 7% of those treated with the silver sulfadiazine could claim the same. Within fifteen days, wounds had healed completely in 87% of the honey group, yet just 10% of the silver sulfadiazine group had healed within the same timeframe. Furthermore, from a cosmetic point of view, there was less noticeable scarring in the group treated with honey. Honey’s antimicrobial property is derived from hydrogen peroxide dissolved within it. Other healing properties are based on honey’s high osmolarity. Honey is a solution comprised mostly of dissolved glucose and fructose within a little bit of water; it is mildly acidic. When applied to wounds, honey’s osmolarity draws fluids up from the wound, producing a dilute layer where nutrients can be passed from honey to tissues and edema is reduced. The reduction of edema allows for better perfusion and oxygenation of tissues is encouraged as a result of a slightly decreased pH in the region. Though gaining attention, honey is still not widely used to treat wounds because of its stigma as a folk remedy [7].
In addition, interest in the use of bacteriophages to treat antibiotic-resistant infections has experienced a renaissance in recent years. A 2007 study by McVay et al. examined the success of P. aeruginosa-specific phages in the prevention of sepsis and mortality in a mouse burn wound model. Subjects were thermally injured and then infected with a lethal dose of P. aeruginosa. Immediately following infection, mice received a phage cocktail intramuscularly, subcutaneously, or intraperitoneally. The mortality rate of the control group was 94%. In stark contrast, mortality was reduced to just 12% when the phage cocktail was administered intraperitoneally. Less, though still significant, success was seen in response to administration of the phages intramuscularly (72% mortality) and subcutaneously (78%).
As of March 2009, the development a phage cocktail against both S. aureus and P. aeruginosa had begun and is in the preliminary stages of clinical testing [6]. It is intended that this cocktail be used as an alternative to or in conjunction with tradition antimicrobial therapies. Further experiments and testing will be required before phage therapies become widespread commercially, yet this remains an exciting and promising step forward in the treatment of burn patients.
Conclusion
Although incidences of mortality and morbidity resulting from burns have declined over the years, particularly after early excision came into popular practice, burn wound infections continue to pose a serious threat to burn victims [3]. The two most prevalent colonizers of burn wounds are Staphylococcus aureus and Pseudomonas areuginosa. Multidrug-resistant microbial infections are becoming increasingly common and difficult to treat. Current research has begun to include the development of alternative therapies to aid patients in recovering from their injuries. In the future, these alternatives may prove useful in treating not only burn infections but other antibiotic-resistant infections as well.
References
1. Atiyeh, B.S., Costagliola, M., Hayek, S.H., Dibo, S.A. (2007). Effect of silver on burn wound infection control and healing: Review of the literature. Burns 33, 139 – 148.
2. “Burn incidence and treatment in the U.S.: 2007 Fact sheet.” (2005). American Burn Association. http://www.ameriburn.org/resources_factsheet.php (Accessed 14 Apr 2009).
3. Church, D., Elsayed, S., Reid, O., Winston, B., Lindsay, R. (2006) Burn Wound Infections. Clinical Microbiology Reviews, 19 (2), 403 – 434.
4. Lyczak, J.B., Cannon, C.L., Pier, G.B. (2000). Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist.Microbes and Infection 2, 1051-1060.
5. McVay, C.S., Velasquez, M., Fralick, J.A. (2007). Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrobial Agents and Chemotherapy 51 (6), 1934 – 1938.
6. Merabishvili, M., Pirnay, J., Verbeken, G., Chanishvili, N., Tediashivili, M., Lashkhi, N., Glonti, T., Kroylov, V., Mast, J., Van Parys, L., Lavigne, R., Volckaert, G., Mattheus, W., Verween, G., De Corte, P., Rose, T., Jennes, S., Zizi, M., De Vos, D., Vaneechoutte, M. (2009). Quality-Controlled Small-Scale Production of a Well-Defined Bacteriophage Cocktail for Use in Human Clinical Trials. PLoS ONE 4(3): e4944.
7. Molan, P.C. (2001). Potential of honey in the treatment of wounds and burns. Am J Cin Dermatol 2 (1), 13 – 19.
8. Murray, C., Hospenthal, D.R. (2008). “Burn Wound Infections”. emedicine http://emedicine.medscape.com/article/213595-overview (Accessed 14 Apr 2009).
9. Safdar, N., Marx, J., Meyer, N.A., Maki, D.G. (2006). Effectiveness of preemptive barrier precautions in controlling nosocomial coloniation and infection by methicillin-resistant Staphylococcus. American Journal of Infection Control October, 476 - 483.
10. Schaber, J.A., Triffo, W.J., Suh, S.J., Oliver, J.W., Hastert, M.C., Griswold, J.A., Auer, M., Hamood, A.N., Rumbaugh, K.P. (2007). Pseudomonas aeruginosa forms biofilms in acute infection independent of cell-to-cell signaling. Infection and Immunity 75 (8), 3715 – 3721.
11. Van Delden, C., Iglewski, B.H. (1998). Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerging Infecetious Diseases 4 (4).
12. Vorvick, L. (2008). Medical encyclopedia: Skin layers. Medline Plus: a service of the U.S. National Library of Medicine and the National Institutes of Health. http://www.nlm.nih.gov/MEDLINEPLUS/ency/imagepages/8912.htm (Accessed Apr 14, 2009).
13. Zetola, N., Francis, J.S., Nuermberger, E.L., Bishai, W.R. (2005). Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis 5, 275 – 286.
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.