Parvovirus B19: Difference between revisions
| (11 intermediate revisions by the same user not shown) | |||
| Line 8: | Line 8: | ||
<i>Parvoviruses</i> are classified in reference to what type of host they are capable of infecting and how they reproduce.<ref name=ncbi/> Those capable of infecting vertebrate hosts are referred to as "Parvovirinae" and are further subdivided by reproduction processes.<ref name=ncbi/> "Parvovirus" refers to members of the <i>Parvoviridae</i> family that reproduce autonomously, but there are also members of this family seen reproducing with a helper virus, "Dependovirus", and preferentially in erythroid cells, "Erythrovirus".<ref name=ncbi/> Parvovirus B19 has recently been found to exhibit extreme specificity for human erythroid progenitor cells, and has therefore been classified as an "erythrovirus".<ref name=rat>[https://www.mdpi.com/2218-273X/11/4/606. Rinkūnaitė, I., Šimoliūnas, E., Bironaitė, D., Rutkienė, R., Bukelskienė, V., Meškys, R., and Bogomolovas, J. "The Effect of a Unique Region of Parvovirus B19 Capsid Protein VP1 on Endothelial Cells" 2021. Biomolecules, 11(4), 606.]</ref><ref name=ncbi/> | <i>Parvoviruses</i> are classified in reference to what type of host they are capable of infecting and how they reproduce.<ref name=ncbi/> Those capable of infecting vertebrate hosts are referred to as "Parvovirinae" and are further subdivided by reproduction processes.<ref name=ncbi/> "Parvovirus" refers to members of the <i>Parvoviridae</i> family that reproduce autonomously, but there are also members of this family seen reproducing with a helper virus, "Dependovirus", and preferentially in erythroid cells, "Erythrovirus".<ref name=ncbi/> Parvovirus B19 has recently been found to exhibit extreme specificity for human erythroid progenitor cells, and has therefore been classified as an "erythrovirus".<ref name=rat>[https://www.mdpi.com/2218-273X/11/4/606. Rinkūnaitė, I., Šimoliūnas, E., Bironaitė, D., Rutkienė, R., Bukelskienė, V., Meškys, R., and Bogomolovas, J. "The Effect of a Unique Region of Parvovirus B19 Capsid Protein VP1 on Endothelial Cells" 2021. Biomolecules, 11(4), 606.]</ref><ref name=ncbi/> | ||
Parvovirus B19V has been implicated in many rheumatologic, neurologic, hepatic, and cardiac disorders.<ref name=Corcioli/> It has classically been considered an acute infection that is quickly cleared by the correct neutralizing antibody production, but B19 DNA has been detectable for many months post infection in many tissues.<ref name=Corcioli/> | |||
==Genome Structure== | ==Genome Structure== | ||
[[Image:B19V_open_reading_frame.png|thumb|300px|left| The genomic map of prototype B19V isolates. This includes 3 open reading frames (ORFs) and their respective nucleotide positions and RNA species a-h with the proteins they code for. Start and stop codons are labeled with their sequence and their nucleotide position. The proteins coded for by e, f, g, and h are unknown. Known proteins are labeled with their names and their sizes in kD.<ref name=Ozawa/> Photo credit: [https://journals-asm-org.libproxy.kenyon.edu/doi/10.1128/jvi.61.8.2395-2406.1987]]] | |||
The <i>Parvovirus</i> genome is a single strand of DNA with 5,596 nucleotides, 4,830 of which are coding regions.<ref name=ncbi/> This region contains two large open reading frames.<ref name=ncbi/> The genome of typical parvoviruses contain two promoters; one on the left for the non-capsid protein, and one in the middle for the two capsid proteins.<ref name=Ozawa>[https://journals-asm-org.libproxy.kenyon.edu/doi/10.1128/jvi.61.8.2395-2406.1987. Ozawa, K., Ayub, J., Hao, Y.S., Kurtzman, G., Shimada, T., and Young, N. "Novel transcription map for the B19 (human) pathogenic parvovirus" 1987. Journal of Virology, 61(8), 2395–2406.]</ref> The B19 parvovirus produces all transcripts starting from a singular promoter on the left side of the genome.<ref name=Ozawa/> There were four RNA species that appeared to have splicing behaviors, and three RNA species terminate in the middle of the genome.<ref name=Ozawa/> The image to the right shows the genome for prototype B19V isolates, and includes the four spliced RNA species, c, d, g, and h.<ref name=Ozawa/> Species c consists of two exons of 0.3 and 1.95 kb, d consists of two exons of 0.2 and 1.95 kb, g consists of two 0.3 kb exons, and h consists of two exons of 0.2 and 0.3 kb.<ref name=Ozawa/> | |||
The prototype genome has two identical inverted terminal repeat sequences that serve as the origin of replication.<ref name=Ros/> These appear in the DNA as hairpin telomeres.<ref name=Ros/> B19V infection was possible in non-permissive cells with the presence of a helper virus and helper virus genes.<ref name=Ros/> Adenovirus genes were seen to transactivate B19V promoters including p44 which is equivalent to the capsid protein promoter in other parvoviruses, but is not used in B19V.<ref name=Ros/> With the transcription of all B19V proteins starting on the left side of the DNA and being created with alternative splicing, it is possible that there was an extension of the VP1u region beyond the typical VP1 of other parvovirus genomes.<ref name=Ros/> This extension is possibly part of the specificity that B19V shows for EPCs.<ref name=Ros/> | |||
[[Image:Screenshot_2024-04-06_at_4.00.04_PM.png|thumb|300px|right| The V9 (A) and A6 (B) genomes are depicted with transcriptional landmarks. These include promoters (ie. P6), splice donors (D), and splice acceptors (A), as well as the ORFs encoded and their predicted protein sizes in kDa. Photo credit: [https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S0042682209005303?via%3Dihub]]] | [[Image:Screenshot_2024-04-06_at_4.00.04_PM.png|thumb|300px|right| The V9 (A) and A6 (B) genomes are depicted with transcriptional landmarks. These include promoters (ie. P6), splice donors (D), and splice acceptors (A), as well as the ORFs encoded and their predicted protein sizes in kDa. Photo credit: [https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S0042682209005303?via%3Dihub]]] | ||
The | The genome has two large open reading frames.<ref name=ncbi/> One large non-structural protein is coded by the left open reading frame, NS1, and the second reading frame codes for two capsid proteins, VP1 and VP2.<ref name=ncbi/><ref name=Ozawa/> The sequences of B19V isolates do not exhibit much genetic variation with NS1 showing incredibly high conservation, and 2-3% divergence in VP1 and VP2 regions.<ref name=ncbi/> When examining isolates from patients with chronic infection due to B19V, there is a much higher degree of variation at both the DNA and protein level.<ref name=Hemauer/> | ||
There are now 3 distinct genotypes recognized as <i>Parvovirus B19</i> including: (1) all prototype B19V isolates, (2) A6 and LaLi isolates, and (3) V9 and related isolates.<ref name=Chen/> These variations in the genome are potentially responsible for the variety of host responses to infection.<ref name=Chen/> In a study on 3 isolates of the 3 genotypes, the A6 isolate did not produce R5, R7, and R9 mRNAs, which are important in the production of VP1 and VP2 as coordinating mRNAs.<ref name=Chen/> There was also an increase in sequence divergence between the 3 isolates. They found that B19V NS1-V9 and B19V NS1-A6 diverge by 13% from B19V NS1, but only diverge by 6% in protein structure.<ref name=Chen/> | There are now 3 distinct genotypes recognized as <i>Parvovirus B19</i> including: (1) all prototype B19V isolates, (2) A6 and LaLi isolates, and (3) V9 and related isolates.<ref name=Chen/> These variations in the genome are potentially responsible for the variety of host responses to infection.<ref name=Chen/> In a study on 3 isolates of the 3 genotypes, the A6 isolate did not produce R5, R7, and R9 mRNAs, which are important in the production of VP1 and VP2 as coordinating mRNAs.<ref name=Chen/> There was also an increase in sequence divergence between the 3 isolates. They found that B19V NS1-V9 and B19V NS1-A6 diverge by 13% from B19V NS1, but only diverge by 6% in protein structure.<ref name=Chen/> | ||
The prevalence of each genotype varies with geography, population, and sample type.<ref name=S-D/> Genotype 1 seems to be the most widely dispersed across the world, but genotype 3 is extremely common in West Africa.<ref name=S-D/> It is thought that genotypes 1 and 2 circulated in North Europe in equal numbers more than 50 years ago, but that genotype 2 is now largely gone from this region.<ref name=S-D/> | |||
==B19V's Essential Proteins== | ==B19V's Essential Proteins== | ||
Parvoviruses are among the smallest DNA containing viruses that are capable of infecting mammal hosts.<ref name=ncbi/> The virion consists of 3 proteins (NS1, VP1, and VP2) and both the positive and negative DNA strands of its singular chromosome.<ref name=ncbi/> The capsid has icosahedral symmetry, and includes 60 copies of the capsomer proteins VP1 and VP2.<ref name=ncbi/> VP2 makes up 96% of the capsid and is encoded from 3125 nt to 4786 nt. VP1 is the minor capsid protein encoded from 2444 nt to 4786 nt. The VP1 coding region is identical to VP2 except for an additional 227 amino acids referred to as the VP1 unique region, or VP1u.<ref name=ncbi/><ref name=Ros>[https://www.mdpi.com/1999-4915/12/12/1463. Ros, Carlos, Bieri, Jan, and Leisi, Remo. "The VP1u of Human Parvovirus B19: A Multifunctional Capsid Protein with Biotechnological Applications" 2020. Viruses, 12(12), 1463.]</ref> | Parvoviruses are among the smallest DNA containing viruses that are capable of infecting mammal hosts.<ref name=ncbi/> The virion consists of 3 proteins (NS1, VP1, and VP2) and both the positive and negative DNA strands of its singular chromosome.<ref name=ncbi/> The capsid has icosahedral symmetry, and includes 60 copies of the capsomer proteins VP1 and VP2.<ref name=ncbi/> VP2 makes up 96% of the capsid and is encoded from 3125 nt to 4786 nt.<ref name=ncbi/> The VP2 major capsid protein shows a “jelly roll” with a Beta barrel motif which can differentiate B19V from other parvoviruses.<ref name=Ros/> VP1 is the minor capsid protein encoded from 2444 nt to 4786 nt.<ref name=ncbi/> The VP1 coding region is identical to VP2 except for an additional 227 amino acids referred to as the VP1 unique region, or VP1u.<ref name=ncbi/><ref name=Ros>[https://www.mdpi.com/1999-4915/12/12/1463. Ros, Carlos, Bieri, Jan, and Leisi, Remo. "The VP1u of Human Parvovirus B19: A Multifunctional Capsid Protein with Biotechnological Applications" 2020. Viruses, 12(12), 1463.]</ref> | ||
VP1u is not accessible to antibodies in native virions, but is accessible in recombinant VP1u.<ref name=Ros/> This could be explained by a compact structural conformational change, or the presence of a masking structure to hide the essential domains from the immune system.<ref name=Ros/> The ability of this region to evade notice by antibodies is important due to its being the immunodominant part of the capsid.<ref name=Ros/> | |||
The predicted structure of the VP1u RBD, between amino acids 5 and 80, contains 3 alpha helices.<ref name=Ros/> These are predicted to occur between amino acids 14 and 31, between amino acids 35 and 45, and between amino acids 59 and 68.<ref name=Ros/> Only one of these helices is conserved across multiple erythroparvoviruses, and has amphiphilic characteristics.<ref name=Ros/> The hydrophobic side of this helix is extremely highly conserved, and point mutations caused loss of viral uptake.<ref name=Ros/> Due to this, it is possible that this helix is indicative of the common binding domain. | |||
The major nonstructural protein coded for by B19V is NS1, which is encoded by nucleotides between 435 and 2448.<ref name=ncbi/> NS1 is thought to contain a DNA binding domain at the N-terminus with conserved motifs for single-strand nickase and an ATP/helicase domain in the center, and a transcription activation at the C-terminal.<ref name=Chen/> For a long time the role of NS1 in infection was largely unknown, but recent studies have highlighted its potential in the proliferation of chronic symptoms related to Parvovirus B19V infection.<ref name=ncbi/><ref name=Jalali>[https://www.hindawi.com/journals/ipid/2022/1639990/. Jalali, Sedigheh, Farhardi, Ali, Dehbidi, G.R., Farjadian, Shirin, Sharifzadeh, Sedigheh, Ranjbaran, Reza, Seyyedi, Noorossadat, Namdari, Sepide and Behzad-Behbahani, Abbas. "The Pathogenic Aspects of Human Parvovirus B19 NS1 Protein in Chronic and Inflammatory Diseases" 2022. Interdisciplinary Perspectives on Infectious Diseases.]</ref> | The major nonstructural protein coded for by B19V is NS1, which is encoded by nucleotides between 435 and 2448.<ref name=ncbi/> NS1 is thought to contain a DNA binding domain at the N-terminus with conserved motifs for single-strand nickase and an ATP/helicase domain in the center, and a transcription activation at the C-terminal.<ref name=Chen/> For a long time the role of NS1 in infection was largely unknown, but recent studies have highlighted its potential in the proliferation of chronic symptoms related to Parvovirus B19V infection.<ref name=ncbi/><ref name=Jalali>[https://www.hindawi.com/journals/ipid/2022/1639990/. Jalali, Sedigheh, Farhardi, Ali, Dehbidi, G.R., Farjadian, Shirin, Sharifzadeh, Sedigheh, Ranjbaran, Reza, Seyyedi, Noorossadat, Namdari, Sepide and Behzad-Behbahani, Abbas. "The Pathogenic Aspects of Human Parvovirus B19 NS1 Protein in Chronic and Inflammatory Diseases" 2022. Interdisciplinary Perspectives on Infectious Diseases.]</ref> | ||
| Line 44: | Line 51: | ||
==Pathogenesis and Infection== | ==Pathogenesis and Infection== | ||
B19V has an extremely high selectivity and cell-type tropism for human erythroid progenitor cells.<ref name=rat/> Infection of endothelial cells is extremely unlikely in genotype 1 B19V's, but has been seen in genotype 2.<ref name=rat/><ref name=Chen/> It has been found that B19V can only successfully replicate in bone-marrow erythroid progenitor cells, or conditions that mirror this setting.<ref name=Chen/><ref name=Zakrzewska>[https://doi.org/10.1016/j.amolm.2023.100007. Zakrzewska, K., Arvia, R., Bua, G., Margheri, F., and Gallinella, G. "Parvovirus B19: Insights and implication for pathogenesis, prevention and therapy" 2023. Aspects of Molecular Medicine.]</ref> | B19V has an extremely high selectivity and cell-type tropism for human erythroid progenitor cells.<ref name=rat/><ref name=Ishida>[https://journals-asm-org.libproxy.kenyon.edu/doi/10.1128/jvi.01631-22. Ishida, K., Noguchi, T., Kimura, S., Suzuki, H., Ebina, H., and Morita, E. "Tracking of Human Parvovirus B19 Virus-Like Particles Using Short Peptide Tags Reveals a Membrane-Associated Extracellular Release of These Particles" 2023. Journal of Virology, 97.]</ref><ref name=Ros/> This tropism is decided at many steps, including receptor-mediated uptake, genome replication, transcription, splicing, and packaging of virus-like particles (VLPs).<ref name=Ros/> It was though that the nuclear export of VLPs was dependent on cell division and mitosis, but with the knowledge that B19V infection induces cell cycle arrest at G2 and M phases, it seems that some VLPs are released during apoptosis.<ref name=Ishida/> The translocation of VLPs to the cytoplasm did show a large increase when cells went through mitosis.<ref name=Ishida/> Infection of endothelial cells is extremely unlikely in genotype 1 B19V's, but has been seen in genotype 2.<ref name=rat/><ref name=Chen/> It has been found that B19V can only successfully replicate in bone-marrow erythroid progenitor cells, or conditions that mirror this setting.<ref name=Chen/><ref name=Zakrzewska>[https://doi.org/10.1016/j.amolm.2023.100007. Zakrzewska, K., Arvia, R., Bua, G., Margheri, F., and Gallinella, G. "Parvovirus B19: Insights and implication for pathogenesis, prevention and therapy" 2023. Aspects of Molecular Medicine.]</ref> The seroprevalence of B19V infection increases with age, from 2-10% at less than 5 years, to 40-60% at age over 20 yearas, and up to 85% in elderly populations.<ref name=S-D/> It is more common in late winter and early summer with a peak of infection every 3 to 4 years.<ref name=S-D/> | ||
[[Image:B19V_viral_uptake.png|thumb|300px|left| B19V recognizes the P antigen, and the VP1u region is moved to the exterior of the capsid. The VP1u region binds to a co-receptor and the capsid enters the cell by endocytosis. The lysosome releases virions into the cytosol which are then transported to the nucleus. In the nucleus, DNA is replicated, mRNA is transcribed and moved to the cytoplasm, and then proteins are made. These proteins are then packaged into capsids which exit the cell and cause the lysis of the original host cell.<ref name=Zakrzewska/> Photo credit: [https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S2949688823000072?via%3Dihub]]] | [[Image:B19V_viral_uptake.png|thumb|300px|left| B19V recognizes the P antigen, and the VP1u region is moved to the exterior of the capsid. The VP1u region binds to a co-receptor and the capsid enters the cell by endocytosis. The lysosome releases virions into the cytosol which are then transported to the nucleus. In the nucleus, DNA is replicated, mRNA is transcribed and moved to the cytoplasm, and then proteins are made. These proteins are then packaged into capsids which exit the cell and cause the lysis of the original host cell.<ref name=Zakrzewska/> Photo credit: [https://www-sciencedirect-com.libproxy.kenyon.edu/science/article/pii/S2949688823000072?via%3Dihub]]] | ||
| Line 52: | Line 59: | ||
As the infection continues, apoptosis and depletion of infected cells leads to erythropoiesis arrest, erythroid aplasia, and the appearance of giant erythroblasts.<ref name=Zakrzewska/> The replication and release of B19V can lead to viremia, with increased viral loads of 1012 virus/mL.<ref name=Zakrzewska/> Infection is normally asymptomatic, but patients with chronic illness, immunodeficiency, and pregnancy can experience much more severe symptoms.<ref name=Zakrzewska/> | As the infection continues, apoptosis and depletion of infected cells leads to erythropoiesis arrest, erythroid aplasia, and the appearance of giant erythroblasts.<ref name=Zakrzewska/> The replication and release of B19V can lead to viremia, with increased viral loads of 1012 virus/mL.<ref name=Zakrzewska/> Infection is normally asymptomatic, but patients with chronic illness, immunodeficiency, and pregnancy can experience much more severe symptoms.<ref name=Zakrzewska/> | ||
B19V is recognized by pathogen-associated molecular pattern molecules and pattern recognition receptors, and exhibits typical prodromal phase production of cytokines.<ref name=Zakrzewska/> There is no imbalance of cytokine patterns in persistent infection, except the cytokine IFN shows increased expression in long-term infection.<ref name=Zakrzewska/> B19V inhibits erythroid cell growth and creates a pile-up of cells in S, G2, and M phases that then go through apoptosis.<ref name=Zakrzewska/> | B19V is recognized by pathogen-associated molecular pattern molecules and pattern recognition receptors, and exhibits typical prodromal phase production of cytokines.<ref name=Zakrzewska/> There is no imbalance of cytokine patterns in persistent infection, except the cytokine IFN shows increased expression in long-term infection.<ref name=Zakrzewska/> B19V inhibits erythroid cell growth and creates a pile-up of cells in S, G2, and M phases that then go through apoptosis.<ref name=Zakrzewska/><ref name=Ishida/> | ||
Antibodies specific to B19V are produced early in infection and typically neutralize symptoms.<ref name=Zakrzewska/> IgM is produced 8-12 days post infection and remains present for 3-6 months, and IgG antibodies are produced a couple of days after IgM production begins.<ref name=Zakrzewska/> As IgM concentrations decrease, IgG antibodies stay present in the body.<ref name=Zakrzewska/> The majority of B19V antibodies are directed against the structural proteins VP1 and VP2.<ref name=Zakrzewska/> Interestingly, antibodies against NS1 protein have been found in 30% of subjects with recent infections, and in those with chronic infections.<ref name=Zakrzewska/> | Antibodies specific to B19V are produced early in infection and typically neutralize symptoms.<ref name=Zakrzewska/> IgM is produced 8-12 days post infection and remains present for 3-6 months, and IgG antibodies are produced a couple of days after IgM production begins.<ref name=Zakrzewska/> As IgM concentrations decrease, IgG antibodies stay present in the body.<ref name=Zakrzewska/> The majority of B19V antibodies are directed against the structural proteins VP1 and VP2.<ref name=Zakrzewska/> Notably, IgM predominantly targets VP2.<ref name=Ros/> Interestingly, antibodies against NS1 protein have been found in 30% of subjects with recent infections, and in those with chronic infections.<ref name=Zakrzewska/> | ||
One very important aspect of B19V pathogenesis is the nuclear import of NS1. Because NS1 is too large to enter through passive diffusion, the IMPα/β nuclear transport protein is necessary.<ref name=Alvisi/> Alvisi et al (2023) designed an experiment to test this using NS1 wild type proteins, an NS1 protein with a K177T substitution, and a mini-genome without the start codon for NS1 in EPO-S1 cells.<ref name=Alvisi/> NS1 proteins were detected 24 hours post transfection in the wild type and K177T substitution cells, but not in cells without the start codon.<ref name=Alvisi/> NS1 was found in the nucleus of cells that had been transfected with wild type NS1, but the K177T substitution NS1 ended up in the cytosol.<ref name=Alvisi/> Viral transcription was found in the wild type cells 24 and 48 hours post transfection, but not in the K177T substitution cells.<ref name=Alvisi/> More research is needed to fully understand the importance of NS1's nuclear location, but it appears to be heavily linked to the ability of the virus to replicate. | One very important aspect of B19V pathogenesis is the nuclear import of NS1. Because NS1 is too large to enter through passive diffusion, the IMPα/β nuclear transport protein is necessary.<ref name=Alvisi/> Alvisi et al (2023) designed an experiment to test this using NS1 wild type proteins, an NS1 protein with a K177T substitution, and a mini-genome without the start codon for NS1 in EPO-S1 cells.<ref name=Alvisi/> NS1 proteins were detected 24 hours post transfection in the wild type and K177T substitution cells, but not in cells without the start codon.<ref name=Alvisi/> NS1 was found in the nucleus of cells that had been transfected with wild type NS1, but the K177T substitution NS1 ended up in the cytosol.<ref name=Alvisi/> Viral transcription was found in the wild type cells 24 and 48 hours post transfection, but not in the K177T substitution cells.<ref name=Alvisi/> More research is needed to fully understand the importance of NS1's nuclear location, but it appears to be heavily linked to the ability of the virus to replicate. | ||
| Line 60: | Line 67: | ||
NS1 has also been found in the cytosol of many B19V infected cells. A common substitution, K177C, is unable to translocate to the nucleus, but appears to retain the ability to induce apoptosis.<ref name=Alvisi/> Therefore, there is a possible functional role of NS1 in the cytoplasm. | NS1 has also been found in the cytosol of many B19V infected cells. A common substitution, K177C, is unable to translocate to the nucleus, but appears to retain the ability to induce apoptosis.<ref name=Alvisi/> Therefore, there is a possible functional role of NS1 in the cytoplasm. | ||
==Potential Use in | While NS1 is important in nuclear uptake, it seems that VP1u has a receptor-binding domain (RBD) that is required for viral uptake into the cell.<ref name=Ros/> This region at the N terminus of the VP1 protein is accessible during interactions with cellular receptors, but does not seem to be recognizable by antibodies.<ref name=Ros/> The RBD spans amino acids 5-80, and capsids without the VP1u were not able to infect target cells.<ref name=Ros/> There is an increasing frequency of neutralizing antibodies found that work against the VP1u region.<ref name=Ros/> It is assumed that B19V uses an erythroid-specific surface molecule as an entry receptor, but it is not quite known what the receptor is.<ref name=Ros/> For a long time, it was assumed that the P antigen is the primary receptor of B19V, but this is an incredibly wide-spread antigen and does not explain the narrow tropism of B19V infection.<ref name=Ros/> While the receptor is not known yet, the RBD in VP1u is known and can explain the tropism seen.<ref name=Ros/> | ||
==Persistent Infection== | |||
B19V DNA has been found in tissues that have been devoid of viremia and IgM antibodies for months.<ref name=Corcioli>[https://onlinelibrary-wiley-com.libproxy.kenyon.edu/doi/10.1002/jmv.21289. Corcioli, F., Zakrzewska, K., Rinieri, A., Fanci, R., Innocenti, M., Civinini, R., De Giorgi, V., Di Lollo, S., and Azzi, A. "Tissue persistence of parvovirus B19 genotypes in asymptomatic persons" 2008. Journal of Medical Virology, 80, 2005-2011.]</ref><ref name=S-D>[https://journals-asm-org.libproxy.kenyon.edu/doi/10.1128/jvi.00684-10.Servant-Delmas, A., Lefrère, J.-J., Morinet, F., and Pillet, S. "Advances in Human B19 Erythrovirus Biology" 2010. Journal of Virology, 84, 9658–9665.]</ref> These long term infections can be asymptomatic or symptomatic depending on the level of infection, and the number of copies of viral DNA present.<ref name=Corcioli/> | |||
It was discovered that asymptomatic B19V persistence is more common in solid tissues than bone marrow tissues, and are much more likely to be members of genotype 2.<ref name=Corcioli/> Persistence in bone marrow was significantly lower than in solid tissues (Chi-squared test, p<0.005).<ref name=Corcioli/> A study by Corcioli et al. (2008) investigated the presence of persistent B19V DNA in 71 tissue samples positive for B19V DNA; they found genotype 1 was found in 20 samples, genotype 2 was found in 48 samples, and genotype 3 was found in 2 samples.<ref name=Corcioli/> This displayed a significant difference between the presence of genotype 1 versus genotype 2 in tissue samples (p<0.01).<ref name=Corcioli/> Anti-B19 antibody IgG was present in 68% of bone marrow tissue samples, 97% of synovium tissue samples, and 58% of skin tissue samples.<ref name=Corcioli/> | |||
In this same study, viral sequences were found in 9 of 44 bone marrow samples, 4 of genotype 1 and 5 of genotype 2.<ref name=Corcioli/> 21 of 38 synovium samples showed viral sequence presence, 5 of genotype 1, 14 of genotype 2, and 1 of genotype 3.<ref name=Corcioli/> There were 29 skin samples that showed positive B19V DNA presence, 11 of genotype 1 and 18 of genotype 2.<ref name=Corcioli/> And 12 of 19 myocardium samples were positive for B19V DNA presence, 11 of genotype 2 and 1 of genotype 3.<ref name=Corcioli/> The copy number of viral DNA ranged from 10 copies per 10,000,000 cells to 700,000 copies per 10,000,000 cells.<ref name=Corcioli/> Roughly 50% of samples had less than 103 copies per 10,000,000 cells.<ref name=Corcioli/> There was a pattern of genotype 2 viral load being lower than genotype 1 load, but it was not a statistically significant difference.<ref name=Corcioli/> The combination of the low viral load and the lack of symptoms shows that the virus persisted, but did not replicate in solid tissues.<ref name=Corcioli/> | |||
Although the virus did not replicate in solid tissues, it is hypothesized that the viral DNA can be reactivated by a helper virus infection.<ref name=Ros/> This could explain the association of B19V with rheumatological disorders, cardiac disorders, and many other long-term conditions.<ref name=Ros/> | |||
==Potential Use in Novel Treatments== | |||
The inhibition of NS1 nuclear transport could result in the inhibition of viral genome replication.<ref name=Alvisi/> The use of IVM significantly reduced the level of NS1 nuclear accumulation at 24h post infection, but the amount of IVM necessary to see this reduction is toxic to healthy cells.<ref name=Alvisi/> Treating cells with IVM or with the introduction of a K177T substitution could decrease viral replication and the observed reduction could be due to the inhibition of NS1 nuclear import.<ref name=Alvisi/> | |||
NS1 nuclear targeting as a potential antiviral intervention | |||
There are various points at which interference could result in restriction of viral replication.<ref name=Zakrzewska/> These include interference before the synthesis of the second DNA strand and the start of macromolecular synthesis, the switch from early pattern expression profile to late pattern expression profile, assembly of viral particles, and the release of infectious virus.<ref name=Zakrzewska/> | |||
There is a possibility of parvovirus particles being used as nanoparticles in drug delivery systems.<ref name=Ishida/> VLP-labeling techniques have shown a high sensitivity for the detection of CLP, and tracking of VLP.<ref name=Ishida/> These findings could be related to developing B19V based protein nanoparticles and high-throughput screening of anti-B19V reagents.<ref name=Ishida/> | |||
Nanocarriers are designed to bring drugs to specific target tissues, and the VP1u region of B19V could provide an effective nanocarrier for drugs designed for erythroid and bone marrow cells.<ref name=Ros/> The addition of VP1u to MS2 capsids detected as few as one erythroleukemic cell in 100,000 isolated white blood cells, which shows an improvement on the detection of hematological disorders and an ability to get earlier diagnoses.<ref name=Ros/> Fluorescent VP1u was unique and sensitive to EPO-dependent erythroid differentiation stages and did not provide too much background noise in detection.<ref name=Ros/> | |||
== | The identification of the cognate receptor will help nanocarrier development to continue, which could be important in treating beta hemoglobin disorders and erythroleukemia.<ref name=Ros/> Beta hemoglobin disorders are a group of diseases that can cause death before 20, and individuals that do live with it need frequent blood transfusions to stay healthy.<ref name=Ros/> An alternate treatment could be the delivery of RNAi therapies to bone marrow cells, which can be done by attaching VP1u to a capsid carrying the required genetic material in oder to target delivery to erythroid cells.<ref name=Ros/> Erythroleukemia is a disease with a mean survival of 8 months.<ref name=Ros/> This disease is treated with chemotherapeutics, but these can be extremely harmful to neighboring healthy cells.<ref name=Ros/> The creation of a vehicle that can efficiently deliver chemotherapies only to erythroid cells will minimize adverse effects for healthy cells.<ref name=Ros/> Because VP1u targets erythroid progenitor cells in the early stages of differentiation and cancer cells are frequently dividing, erythrocancer cells are the ideal host for B19V.<ref name=Ros/> One major road block to this avenue of using B19V as a nanocarrier is that many people have innate immunity against B19V infection, but it is possible that using a short peptide of just the RBD of VP1u could escape antibody notice and show effective drug delivery.<ref name=Ros/> | ||
==References== | ==References== | ||
<references /> | <references /> | ||
<br><br>Authored for BIOL 238 Microbiology, taught by [https://biology.kenyon.edu/slonc/slonc.htm Joan Slonczewski,]at [http://www.kenyon.edu/index.xml Kenyon College,]2024 | <br><br>Authored for BIOL 238 Microbiology, taught by [https://biology.kenyon.edu/slonc/slonc.htm Joan Slonczewski,]at [http://www.kenyon.edu/index.xml Kenyon College,]2024 | ||
Latest revision as of 02:06, 15 April 2024
Background
By Grace Potter
Parvovirus B19 is the only member of the Parvoviridae family that has been found to infect human hosts.[1] It was discovered in 1974, when a research group looking at hepatitis B surface antigens found a serum sample with unexpected results.[1] Another lab in Japan described a similar virus in 1979 that they called "Nakatami".[1] When compared, the two were found to be identical.[1] In 1985 this virus was officially recognized as a member of the Parvoviridae family due to its similarities in genome size and density.[1]
Infection by Parvovirus B19 (Parvo B19V) causes many diseases, including "fifth disease" in children, aplastic crisis for people with hemolytic anemia, anemia in immunocompromised patients, acute or chronic arthropathy in adults, and fetal hydrops in pregnant women.[2][3] Changes in the genome are potentially responsible for the wide variation of clinical presentations associated with B19V infection.[2]
Parvoviruses are classified in reference to what type of host they are capable of infecting and how they reproduce.[1] Those capable of infecting vertebrate hosts are referred to as "Parvovirinae" and are further subdivided by reproduction processes.[1] "Parvovirus" refers to members of the Parvoviridae family that reproduce autonomously, but there are also members of this family seen reproducing with a helper virus, "Dependovirus", and preferentially in erythroid cells, "Erythrovirus".[1] Parvovirus B19 has recently been found to exhibit extreme specificity for human erythroid progenitor cells, and has therefore been classified as an "erythrovirus".[4][1]
Parvovirus B19V has been implicated in many rheumatologic, neurologic, hepatic, and cardiac disorders.[5] It has classically been considered an acute infection that is quickly cleared by the correct neutralizing antibody production, but B19 DNA has been detectable for many months post infection in many tissues.[5]
Genome Structure

The Parvovirus genome is a single strand of DNA with 5,596 nucleotides, 4,830 of which are coding regions.[1] This region contains two large open reading frames.[1] The genome of typical parvoviruses contain two promoters; one on the left for the non-capsid protein, and one in the middle for the two capsid proteins.[6] The B19 parvovirus produces all transcripts starting from a singular promoter on the left side of the genome.[6] There were four RNA species that appeared to have splicing behaviors, and three RNA species terminate in the middle of the genome.[6] The image to the right shows the genome for prototype B19V isolates, and includes the four spliced RNA species, c, d, g, and h.[6] Species c consists of two exons of 0.3 and 1.95 kb, d consists of two exons of 0.2 and 1.95 kb, g consists of two 0.3 kb exons, and h consists of two exons of 0.2 and 0.3 kb.[6]
The prototype genome has two identical inverted terminal repeat sequences that serve as the origin of replication.[7] These appear in the DNA as hairpin telomeres.[7] B19V infection was possible in non-permissive cells with the presence of a helper virus and helper virus genes.[7] Adenovirus genes were seen to transactivate B19V promoters including p44 which is equivalent to the capsid protein promoter in other parvoviruses, but is not used in B19V.[7] With the transcription of all B19V proteins starting on the left side of the DNA and being created with alternative splicing, it is possible that there was an extension of the VP1u region beyond the typical VP1 of other parvovirus genomes.[7] This extension is possibly part of the specificity that B19V shows for EPCs.[7]

The genome has two large open reading frames.[1] One large non-structural protein is coded by the left open reading frame, NS1, and the second reading frame codes for two capsid proteins, VP1 and VP2.[1][6] The sequences of B19V isolates do not exhibit much genetic variation with NS1 showing incredibly high conservation, and 2-3% divergence in VP1 and VP2 regions.[1] When examining isolates from patients with chronic infection due to B19V, there is a much higher degree of variation at both the DNA and protein level.[3]
There are now 3 distinct genotypes recognized as Parvovirus B19 including: (1) all prototype B19V isolates, (2) A6 and LaLi isolates, and (3) V9 and related isolates.[2] These variations in the genome are potentially responsible for the variety of host responses to infection.[2] In a study on 3 isolates of the 3 genotypes, the A6 isolate did not produce R5, R7, and R9 mRNAs, which are important in the production of VP1 and VP2 as coordinating mRNAs.[2] There was also an increase in sequence divergence between the 3 isolates. They found that B19V NS1-V9 and B19V NS1-A6 diverge by 13% from B19V NS1, but only diverge by 6% in protein structure.[2]
The prevalence of each genotype varies with geography, population, and sample type.[8] Genotype 1 seems to be the most widely dispersed across the world, but genotype 3 is extremely common in West Africa.[8] It is thought that genotypes 1 and 2 circulated in North Europe in equal numbers more than 50 years ago, but that genotype 2 is now largely gone from this region.[8]
B19V's Essential Proteins
Parvoviruses are among the smallest DNA containing viruses that are capable of infecting mammal hosts.[1] The virion consists of 3 proteins (NS1, VP1, and VP2) and both the positive and negative DNA strands of its singular chromosome.[1] The capsid has icosahedral symmetry, and includes 60 copies of the capsomer proteins VP1 and VP2.[1] VP2 makes up 96% of the capsid and is encoded from 3125 nt to 4786 nt.[1] The VP2 major capsid protein shows a “jelly roll” with a Beta barrel motif which can differentiate B19V from other parvoviruses.[7] VP1 is the minor capsid protein encoded from 2444 nt to 4786 nt.[1] The VP1 coding region is identical to VP2 except for an additional 227 amino acids referred to as the VP1 unique region, or VP1u.[1][7]
VP1u is not accessible to antibodies in native virions, but is accessible in recombinant VP1u.[7] This could be explained by a compact structural conformational change, or the presence of a masking structure to hide the essential domains from the immune system.[7] The ability of this region to evade notice by antibodies is important due to its being the immunodominant part of the capsid.[7]
The predicted structure of the VP1u RBD, between amino acids 5 and 80, contains 3 alpha helices.[7] These are predicted to occur between amino acids 14 and 31, between amino acids 35 and 45, and between amino acids 59 and 68.[7] Only one of these helices is conserved across multiple erythroparvoviruses, and has amphiphilic characteristics.[7] The hydrophobic side of this helix is extremely highly conserved, and point mutations caused loss of viral uptake.[7] Due to this, it is possible that this helix is indicative of the common binding domain.
The major nonstructural protein coded for by B19V is NS1, which is encoded by nucleotides between 435 and 2448.[1] NS1 is thought to contain a DNA binding domain at the N-terminus with conserved motifs for single-strand nickase and an ATP/helicase domain in the center, and a transcription activation at the C-terminal.[2] For a long time the role of NS1 in infection was largely unknown, but recent studies have highlighted its potential in the proliferation of chronic symptoms related to Parvovirus B19V infection.[1][9]
Results from a study indicate that the transfection of hPVB19 NS1 into HEK-293T cells increased proinflammatory cytokine levels.[9] This is consistent with the findings that NS1 might play a role in facilitating the upregulation of inflammatory reactions, including chronic arthritis which can be a symptom of chronic B19V infection.[9]
The figure to the right indicates a significant increase in the expression levels of the cytokines IL-17A and TNF-α. IL-17A and TNF-α initiate inflammation cascades in host cells.[9] Inflammation has been found to increase the risk of cells becoming carcinogenic, and further research is needed to assess the role of these cytokines in cancer development.[9] An increase in IFN and TNF in supernatants from NS1-transfected cells can induce the expression of IL-6, an important proinflammatory cytokine, and shows the potential of NS1 in triggering autoimmune disorders.[9] Further research is needed to fully understand the impact of NS1 on autoimmune disorders, especially considering its impact on proinflammatory cytokines.
Differences in the NS1 proteins found across the 3 genotypes of Parvovirus B19 show that B19V NS1-V9 and B19V NS1-A6 diverge by 13% from B19V NS1 in genetic sequence, but only diverge by 6% in protein structure.[2] The inability of B19V NS1-A6 to replicate the prototype B19V genome in comparison with its ability to replicate and package other A6 genomes, shows that the NS1 protein has genotype specific activity.[2]
Of the proteins produced by Parvovirus B19, only NS1 is predicted to possess a classical nuclear localization signal (cNLSL).[10] NS1 becomes detectable at low levels 6 hours post infection, and peaks 24 to 36 hours post infection.[10] Confocal laser scanning microscopy confirmed that NS1 accumulated in the nucleus.[10] More on the nuclear localization of NS1 in the pathogenesis and infectivity section.
In many parvoviruses, the NS1 protein contains a cNLS sequence.[10] Some of the cNLSs are more similar to each other than others, and overall these DNA and amino acid sequences are not highly conserved.[10] The parvovirus minute virus of mice and ungulate protoparvovius 1 both have bipartite cNLSs, but human parvovirus B19 has a monopartite cNLS.[10] Bipartite cNLSs are more efficient at binding the IMP nuclear translocation protein, so the presence of a monopartite cNLS could be an effort to limit NS1 levels in the nucleus.[10] This seems to provide evidence for the independent evolution of cNLS sequences, and therefore the importance of the NS1 protein entering the nucleus in the viral life cycle.[10]
There are other open reading frames in the B19V genome, but the function of their resulting proteins is largely unknown.[1] Further research is needed in this area.
Pathogenesis and Infection
B19V has an extremely high selectivity and cell-type tropism for human erythroid progenitor cells.[4][11][7] This tropism is decided at many steps, including receptor-mediated uptake, genome replication, transcription, splicing, and packaging of virus-like particles (VLPs).[7] It was though that the nuclear export of VLPs was dependent on cell division and mitosis, but with the knowledge that B19V infection induces cell cycle arrest at G2 and M phases, it seems that some VLPs are released during apoptosis.[11] The translocation of VLPs to the cytoplasm did show a large increase when cells went through mitosis.[11] Infection of endothelial cells is extremely unlikely in genotype 1 B19V's, but has been seen in genotype 2.[4][2] It has been found that B19V can only successfully replicate in bone-marrow erythroid progenitor cells, or conditions that mirror this setting.[2][12] The seroprevalence of B19V infection increases with age, from 2-10% at less than 5 years, to 40-60% at age over 20 yearas, and up to 85% in elderly populations.[8] It is more common in late winter and early summer with a peak of infection every 3 to 4 years.[8]
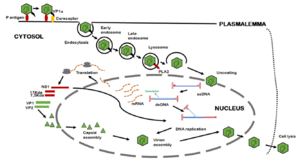
B19V's path of infection starts with entrance through the respiratory tract, and from there it reaches the bloodstream and eventually the bone marrow.[12] Interactions between the bone-marrow erythroid progenitor cells glycolipid globosides, blood group P antigen, and an unknown secondary receptor allow the viral particle to enter the cell.[12] The interaction between the viral shell and P antigen leads to the externalization of the unique region of VP1, which consequently allows B19V to enter the cell through an endocytic pathway.[12] The VP1u region exhibits phospholipase A2 activity, which allows the virus to evade lysosomal fusion and ensure infectivity when it is exposed.[12]
As the infection continues, apoptosis and depletion of infected cells leads to erythropoiesis arrest, erythroid aplasia, and the appearance of giant erythroblasts.[12] The replication and release of B19V can lead to viremia, with increased viral loads of 1012 virus/mL.[12] Infection is normally asymptomatic, but patients with chronic illness, immunodeficiency, and pregnancy can experience much more severe symptoms.[12]
B19V is recognized by pathogen-associated molecular pattern molecules and pattern recognition receptors, and exhibits typical prodromal phase production of cytokines.[12] There is no imbalance of cytokine patterns in persistent infection, except the cytokine IFN shows increased expression in long-term infection.[12] B19V inhibits erythroid cell growth and creates a pile-up of cells in S, G2, and M phases that then go through apoptosis.[12][11]
Antibodies specific to B19V are produced early in infection and typically neutralize symptoms.[12] IgM is produced 8-12 days post infection and remains present for 3-6 months, and IgG antibodies are produced a couple of days after IgM production begins.[12] As IgM concentrations decrease, IgG antibodies stay present in the body.[12] The majority of B19V antibodies are directed against the structural proteins VP1 and VP2.[12] Notably, IgM predominantly targets VP2.[7] Interestingly, antibodies against NS1 protein have been found in 30% of subjects with recent infections, and in those with chronic infections.[12]
One very important aspect of B19V pathogenesis is the nuclear import of NS1. Because NS1 is too large to enter through passive diffusion, the IMPα/β nuclear transport protein is necessary.[10] Alvisi et al (2023) designed an experiment to test this using NS1 wild type proteins, an NS1 protein with a K177T substitution, and a mini-genome without the start codon for NS1 in EPO-S1 cells.[10] NS1 proteins were detected 24 hours post transfection in the wild type and K177T substitution cells, but not in cells without the start codon.[10] NS1 was found in the nucleus of cells that had been transfected with wild type NS1, but the K177T substitution NS1 ended up in the cytosol.[10] Viral transcription was found in the wild type cells 24 and 48 hours post transfection, but not in the K177T substitution cells.[10] More research is needed to fully understand the importance of NS1's nuclear location, but it appears to be heavily linked to the ability of the virus to replicate.
NS1 has also been found in the cytosol of many B19V infected cells. A common substitution, K177C, is unable to translocate to the nucleus, but appears to retain the ability to induce apoptosis.[10] Therefore, there is a possible functional role of NS1 in the cytoplasm.
While NS1 is important in nuclear uptake, it seems that VP1u has a receptor-binding domain (RBD) that is required for viral uptake into the cell.[7] This region at the N terminus of the VP1 protein is accessible during interactions with cellular receptors, but does not seem to be recognizable by antibodies.[7] The RBD spans amino acids 5-80, and capsids without the VP1u were not able to infect target cells.[7] There is an increasing frequency of neutralizing antibodies found that work against the VP1u region.[7] It is assumed that B19V uses an erythroid-specific surface molecule as an entry receptor, but it is not quite known what the receptor is.[7] For a long time, it was assumed that the P antigen is the primary receptor of B19V, but this is an incredibly wide-spread antigen and does not explain the narrow tropism of B19V infection.[7] While the receptor is not known yet, the RBD in VP1u is known and can explain the tropism seen.[7]
Persistent Infection
B19V DNA has been found in tissues that have been devoid of viremia and IgM antibodies for months.[5][8] These long term infections can be asymptomatic or symptomatic depending on the level of infection, and the number of copies of viral DNA present.[5]
It was discovered that asymptomatic B19V persistence is more common in solid tissues than bone marrow tissues, and are much more likely to be members of genotype 2.[5] Persistence in bone marrow was significantly lower than in solid tissues (Chi-squared test, p<0.005).[5] A study by Corcioli et al. (2008) investigated the presence of persistent B19V DNA in 71 tissue samples positive for B19V DNA; they found genotype 1 was found in 20 samples, genotype 2 was found in 48 samples, and genotype 3 was found in 2 samples.[5] This displayed a significant difference between the presence of genotype 1 versus genotype 2 in tissue samples (p<0.01).[5] Anti-B19 antibody IgG was present in 68% of bone marrow tissue samples, 97% of synovium tissue samples, and 58% of skin tissue samples.[5]
In this same study, viral sequences were found in 9 of 44 bone marrow samples, 4 of genotype 1 and 5 of genotype 2.[5] 21 of 38 synovium samples showed viral sequence presence, 5 of genotype 1, 14 of genotype 2, and 1 of genotype 3.[5] There were 29 skin samples that showed positive B19V DNA presence, 11 of genotype 1 and 18 of genotype 2.[5] And 12 of 19 myocardium samples were positive for B19V DNA presence, 11 of genotype 2 and 1 of genotype 3.[5] The copy number of viral DNA ranged from 10 copies per 10,000,000 cells to 700,000 copies per 10,000,000 cells.[5] Roughly 50% of samples had less than 103 copies per 10,000,000 cells.[5] There was a pattern of genotype 2 viral load being lower than genotype 1 load, but it was not a statistically significant difference.[5] The combination of the low viral load and the lack of symptoms shows that the virus persisted, but did not replicate in solid tissues.[5]
Although the virus did not replicate in solid tissues, it is hypothesized that the viral DNA can be reactivated by a helper virus infection.[7] This could explain the association of B19V with rheumatological disorders, cardiac disorders, and many other long-term conditions.[7]
Potential Use in Novel Treatments
The inhibition of NS1 nuclear transport could result in the inhibition of viral genome replication.[10] The use of IVM significantly reduced the level of NS1 nuclear accumulation at 24h post infection, but the amount of IVM necessary to see this reduction is toxic to healthy cells.[10] Treating cells with IVM or with the introduction of a K177T substitution could decrease viral replication and the observed reduction could be due to the inhibition of NS1 nuclear import.[10]
NS1 nuclear targeting as a potential antiviral intervention
There are various points at which interference could result in restriction of viral replication.[12] These include interference before the synthesis of the second DNA strand and the start of macromolecular synthesis, the switch from early pattern expression profile to late pattern expression profile, assembly of viral particles, and the release of infectious virus.[12]
There is a possibility of parvovirus particles being used as nanoparticles in drug delivery systems.[11] VLP-labeling techniques have shown a high sensitivity for the detection of CLP, and tracking of VLP.[11] These findings could be related to developing B19V based protein nanoparticles and high-throughput screening of anti-B19V reagents.[11]
Nanocarriers are designed to bring drugs to specific target tissues, and the VP1u region of B19V could provide an effective nanocarrier for drugs designed for erythroid and bone marrow cells.[7] The addition of VP1u to MS2 capsids detected as few as one erythroleukemic cell in 100,000 isolated white blood cells, which shows an improvement on the detection of hematological disorders and an ability to get earlier diagnoses.[7] Fluorescent VP1u was unique and sensitive to EPO-dependent erythroid differentiation stages and did not provide too much background noise in detection.[7]
The identification of the cognate receptor will help nanocarrier development to continue, which could be important in treating beta hemoglobin disorders and erythroleukemia.[7] Beta hemoglobin disorders are a group of diseases that can cause death before 20, and individuals that do live with it need frequent blood transfusions to stay healthy.[7] An alternate treatment could be the delivery of RNAi therapies to bone marrow cells, which can be done by attaching VP1u to a capsid carrying the required genetic material in oder to target delivery to erythroid cells.[7] Erythroleukemia is a disease with a mean survival of 8 months.[7] This disease is treated with chemotherapeutics, but these can be extremely harmful to neighboring healthy cells.[7] The creation of a vehicle that can efficiently deliver chemotherapies only to erythroid cells will minimize adverse effects for healthy cells.[7] Because VP1u targets erythroid progenitor cells in the early stages of differentiation and cancer cells are frequently dividing, erythrocancer cells are the ideal host for B19V.[7] One major road block to this avenue of using B19V as a nanocarrier is that many people have innate immunity against B19V infection, but it is possible that using a short peptide of just the RBD of VP1u could escape antibody notice and show effective drug delivery.[7]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 Heegaard, E.D. and Brown, K.E. "Human Parvovirus B19." 2002. Clinical Microbiology Review 15(3):485-505.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Chen, Z., Guan, W., Cheng, F., Chen, A.Y., and Qiu, J. "Molecular characterization of human parvovirus B19 genotypes 2 and 3" 2009. Virology, 394(2), 276-285.
- ↑ 3.0 3.1 Hemauer, A., von Poblotzki, A., Gigler, A., Cassinotti, P., Siegl, G., Wolf, H., and Modrow, S. "Sequence variability among different parvovirus B19 isolates" 1996. Journal of General Virology, 77(8), 1781-1785.
- ↑ 4.0 4.1 4.2 Rinkūnaitė, I., Šimoliūnas, E., Bironaitė, D., Rutkienė, R., Bukelskienė, V., Meškys, R., and Bogomolovas, J. "The Effect of a Unique Region of Parvovirus B19 Capsid Protein VP1 on Endothelial Cells" 2021. Biomolecules, 11(4), 606.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 Corcioli, F., Zakrzewska, K., Rinieri, A., Fanci, R., Innocenti, M., Civinini, R., De Giorgi, V., Di Lollo, S., and Azzi, A. "Tissue persistence of parvovirus B19 genotypes in asymptomatic persons" 2008. Journal of Medical Virology, 80, 2005-2011.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Ozawa, K., Ayub, J., Hao, Y.S., Kurtzman, G., Shimada, T., and Young, N. "Novel transcription map for the B19 (human) pathogenic parvovirus" 1987. Journal of Virology, 61(8), 2395–2406.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 7.20 7.21 7.22 7.23 7.24 7.25 7.26 7.27 7.28 7.29 7.30 7.31 7.32 7.33 7.34 7.35 7.36 7.37 Ros, Carlos, Bieri, Jan, and Leisi, Remo. "The VP1u of Human Parvovirus B19: A Multifunctional Capsid Protein with Biotechnological Applications" 2020. Viruses, 12(12), 1463.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 A., Lefrère, J.-J., Morinet, F., and Pillet, S. "Advances in Human B19 Erythrovirus Biology" 2010. Journal of Virology, 84, 9658–9665.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Jalali, Sedigheh, Farhardi, Ali, Dehbidi, G.R., Farjadian, Shirin, Sharifzadeh, Sedigheh, Ranjbaran, Reza, Seyyedi, Noorossadat, Namdari, Sepide and Behzad-Behbahani, Abbas. "The Pathogenic Aspects of Human Parvovirus B19 NS1 Protein in Chronic and Inflammatory Diseases" 2022. Interdisciplinary Perspectives on Infectious Diseases.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 10.12 10.13 10.14 10.15 10.16 Alvisi, G., Manaresi, E., Cross, E.M., Hoad, M., Akbari, N., Pavan, S., Ariawan, D., Bua, G., Petersen, G.F., Forwood, J., and Gallinella, G. "Importin α/β-dependent nuclear transport of human parvovirus B19 nonstructural protein 1 is essential for viral replication" 2023. Antiviral Research, 213.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Ishida, K., Noguchi, T., Kimura, S., Suzuki, H., Ebina, H., and Morita, E. "Tracking of Human Parvovirus B19 Virus-Like Particles Using Short Peptide Tags Reveals a Membrane-Associated Extracellular Release of These Particles" 2023. Journal of Virology, 97.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 12.14 12.15 12.16 12.17 12.18 Zakrzewska, K., Arvia, R., Bua, G., Margheri, F., and Gallinella, G. "Parvovirus B19: Insights and implication for pathogenesis, prevention and therapy" 2023. Aspects of Molecular Medicine.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024

