Sphingomonas sp., agents: Difference between revisions
No edit summary |
|||
| Line 15: | Line 15: | ||
=Environmental Contamination: The Issue= | |||
Polycyclic aromatic hydrocarbons (PAHs) are a pressing issue for several reasons, the main being that they are dangerous for both humans and other organisms. PAHs are a major component of many fuels, and therefore enter the environment through vehicle leaks, incorrect disposal of wastes, and oil spills. These compounds are extremely stable thanks to a system of double bonds throughout their hydrocarbon rings, and are therefore highly resilient in the environment. Finally, PAHs have low bioavailability and can be used as carbon sources by only a select few organisms. | |||
An additional problem is that many bacteria cannot degrade chemicals unless they are dissolved in water. Because of their high concentrations of hydrophobic carbon-hydrogen bonds, however, PAHs interact with non-aqueous media and soil organic matter. This often makes them unavailable to degradation and only furthers their persistence. | |||
=Diverse Specializations of <i>Sphingomonas</i> Species= | =Diverse Specializations of <i>Sphingomonas</i> Species= | ||
| Line 35: | Line 41: | ||
Often, soil around nuclear reactors and power plants or uranium mines contains uranium (IV) in forms such as [UO2(CO3)2]<sup>−2</sup> and [UO2(CO3)3]<sup>−4</sup>, which can permeate groundwater and lead to health hazards. The <i>Sphingomonas</i> strain BSAR-1 was found to precipitate uranium thanks to an alkaline phosphatase secreted into the outside medium, thus preventing hazards. When the gene encoding this function, dubbed <i>phoK</i>, was inserted into <i>E. coli</i> and overexpressed, the bacteria became powerful uranium precipitators. This recombinant strain precipitated more than 90% of a provided quantity of uranium in less than 2 hrs, whereas the original <i>Sphingomonas</i> degraded this quantity in a little over 7 hrs. This study revealed both certain <i>Sphingomonas</i> species’ facility at bioprecipiation of contaminants, and the possibility that these traits could be transferred to other species to be used in bioremediation. | Often, soil around nuclear reactors and power plants or uranium mines contains uranium (IV) in forms such as [UO2(CO3)2]<sup>−2</sup> and [UO2(CO3)3]<sup>−4</sup>, which can permeate groundwater and lead to health hazards. The <i>Sphingomonas</i> strain BSAR-1 was found to precipitate uranium thanks to an alkaline phosphatase secreted into the outside medium, thus preventing hazards. When the gene encoding this function, dubbed <i>phoK</i>, was inserted into <i>E. coli</i> and overexpressed, the bacteria became powerful uranium precipitators. This recombinant strain precipitated more than 90% of a provided quantity of uranium in less than 2 hrs, whereas the original <i>Sphingomonas</i> degraded this quantity in a little over 7 hrs. This study revealed both certain <i>Sphingomonas</i> species’ facility at bioprecipiation of contaminants, and the possibility that these traits could be transferred to other species to be used in bioremediation. | ||
(http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2546639/?tool=pmcentrez) | (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2546639/?tool=pmcentrez) | ||
=Past Utilization of <i>Sphingomonas</i>= | =Past Utilization of <i>Sphingomonas</i>= | ||
Revision as of 09:47, 25 April 2011
Introduction
The Genus Sphingomonas includes a range of bacterium that are remarkable for their ability to break down polycyclic hydrocarbons. Bacteria in this genus have been detected in a variety of environments, both marine and terrestrial. In recent years, these bacteria have been a focus of study because of their possible applications for bioremediation (one of the major components of oil being stable aromatic hydrocarbons). [CITATION]
Characterization of Sphingomonas
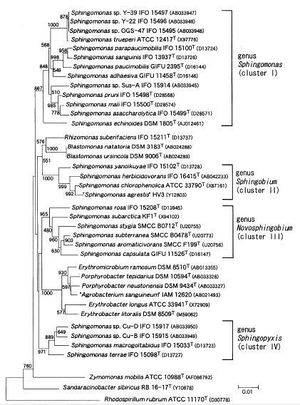
The Genus Sphingomonas includes many bacteria with varied morphological traits. Though initially only a few were none and mostly in a clinical setting, further studies started to reveal many morphologically similar strains. For this reason, in 2001 they were separated into four distinct clusters within the Genus (Figure 2). These clusters, collectively known as the Sphingomonads, are individually
known as Sphingomonas, Sphingobium, Novosphingobium, and Sphingopyxis. There are more than 20 known species that are distributed amongst these strains according to their chemotaxonomic and phenotypic traits, as well as their 16S rRNA gene sequences. (http://ijs.sgmjournals.org/cgi/content/abstract/51/4/1405)
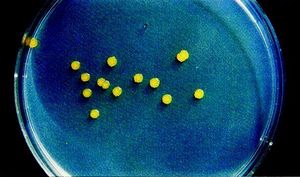
Some shared traits among all strains of Sphingomonas are that they are Gram-negative and possess a bacillus (rod) shape. The bacteria are also strictly aerobic chemoheterophs. Whereas other bacteria utilize lipopolysaccharides in their cell envelopes, Sphingomonas integrate glycosphingolipids. This is the primary distinguishing characteristic separating them from other species in the α-subclass of Proteobacteria. In cultures Sphingomonas colonies typically appear yellow (Figure 3). Finally, Sphingomonas species’ major quinone is characteristically ubiquinone 10. (http://www.springerlink.com/content/v3654176x2651434/)
Environmental Contamination: The Issue
Polycyclic aromatic hydrocarbons (PAHs) are a pressing issue for several reasons, the main being that they are dangerous for both humans and other organisms. PAHs are a major component of many fuels, and therefore enter the environment through vehicle leaks, incorrect disposal of wastes, and oil spills. These compounds are extremely stable thanks to a system of double bonds throughout their hydrocarbon rings, and are therefore highly resilient in the environment. Finally, PAHs have low bioavailability and can be used as carbon sources by only a select few organisms.
An additional problem is that many bacteria cannot degrade chemicals unless they are dissolved in water. Because of their high concentrations of hydrophobic carbon-hydrogen bonds, however, PAHs interact with non-aqueous media and soil organic matter. This often makes them unavailable to degradation and only furthers their persistence.
Diverse Specializations of Sphingomonas Species
Though Sphingomonas species vary in their ideal habitats, most seem to possess functions useful to bioremediation. Therefore, much research has been focused on finding strains that thrive under conditions where bioremediation may be necessary. Some examples include:
Subsurface Soils
A 1995 study collected bacteria from the United States Southeast Coastal Plain subsurface sediments and subjected them to a battery of tests. Tests of phylogeny using 16S rRNA genes showed that some of the collected strains were closely related to Sphingomonas capsulate. Additionally, it was found that these bacteria contained sphingolipids (characteristic of Sphingomonas). Perhaps most notably, most of these bacteria were able to grow on a variety of aromatic compounds, including both toluene and naphthalene. Though many other gram-negative heterotrophic aerobic bacteria can degrade these compounds, it is rare that one organism can catabolize both. Of the strains collected, one could degrade salicylate and benzoate, two could degrade p-cresol and salicylate, and five strains could degrade flourene, biphenyl, and dibenzothiophene. Though further characterization of these strains is ongoing, they are believed to be members of Genus Sphingomonas, all specializing in catabolism of different compounds at the subsurface level. (http://aem.asm.org/cgi/reprint/61/5/1917)
Cold Soils
At Scott Base in Antarctica, a strain known as Ant 17 was isolated from fuel-contaminated soil. The bacteria grows from temperatures as low as 1oC to 35oC, and can mineralize phenanthrene over a wider range than 24oC. Despite the cold conditions, this strain appeared to be able to metabolize at a rate quicker than should be allowed by mesophilic enzyme kinetics. Indeed, Ant 17 was more effective than Sphingomonas spp. WPO-1 under UV-irradiation and freeze-thaw cycles, indicative of adaptation to the Antarctic environment. Most relevant to bioremediation was the fact that Ant 17 could degrade the aromatic portions of various crude oils, jet fuel, and diesel fuel. Due to its wide aromatic substrate range and ability to catabolize them in low, fluctuating temperatures, Ant 17 appears to be a promising bioremedial microbe for the cleaning of cold, fuel-contaminated environments. (http://www.springerlink.com/content/nrklbqnvthatxjyl/)
Aquatic Environments
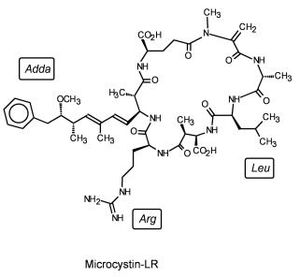
In China a study was performed to assess the abilities of many marine lifeforms to break down microcystin, a common marine contaminant (Figure 4). Among the plants, invertebrates, fish, and bacteria whose abilities were studied was Sphingomonas sp. Sphingomonas was able to catabolize microcystin-LR using three different intracellular hydrolytic enzymes, one of which came to be known as microxystinase. This study represents an avenue for aquatic bioremediation. (http://en.cnki.com.cn/Article_en/CJFDTOTAL-STKX200501025.htm)
Uranium-Contaminated Environments
Often, soil around nuclear reactors and power plants or uranium mines contains uranium (IV) in forms such as [UO2(CO3)2]−2 and [UO2(CO3)3]−4, which can permeate groundwater and lead to health hazards. The Sphingomonas strain BSAR-1 was found to precipitate uranium thanks to an alkaline phosphatase secreted into the outside medium, thus preventing hazards. When the gene encoding this function, dubbed phoK, was inserted into E. coli and overexpressed, the bacteria became powerful uranium precipitators. This recombinant strain precipitated more than 90% of a provided quantity of uranium in less than 2 hrs, whereas the original Sphingomonas degraded this quantity in a little over 7 hrs. This study revealed both certain Sphingomonas species’ facility at bioprecipiation of contaminants, and the possibility that these traits could be transferred to other species to be used in bioremediation. (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2546639/?tool=pmcentrez)
Past Utilization of Sphingomonas
AHAHHHHHHH!!!
Pathological Implications
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2010, Kenyon College.