|
|
| (20 intermediate revisions by the same user not shown) |
| Line 1: |
Line 1: |
| Granulicatella adiacens
| | ==<i>Brevibacillus laterosporus</i>, a bacterial biological control agent of Western Corn Rootworm.== |
|
| |
|
| Lilian Sool-Esol
| | ==Background== |
| MicrobeWiki
| | [[Image:PHIL_22882_lores.jpg|thumb|300px|right|This illustration depicts a three-dimensional (3D), computer-generated image, of a group of Gram-positive, Streptococcus agalactiae (group B Streptococcus) bacteria. The photo credit for this image belongs to Alissa Eckert, who is a medical illustrator at the [http://www.cdc.gov/ CDC].]] |
| Prof. Angela Hahn
| | <br>By Katya Naphtali<br> |
| 16 December, 2013
| |
| Granulicatella adiacens
| |
|
| |
|
| In 1961, Frenkel and Hirsch were the first to describe the Granulicatella bacteria genus as a nutritionally variant streptococcus (NVS) (Christensen et al., 2001). The Granulicatella genus is known to be a normal flora of the upper respiratory, gastrointestinal and urogenital tracts of humans. Normal flora is a microorganism that normally resides at a given site and under normal circumstances does not cause disease. Granulicatella adiacens whose genus name was formerly known as Abiotrophia because the bacteria genus is believed to be nutritionally deficient and even when samples are strained in the laboratory, a supplemented media with rich agar is used to culture samples of these bacteria. The word “Abiotrophia” means life nutrition deficiency (Bizzarro et al., 2011). Granulicatella adiacens, a species of this genus is found in the oral cavity, intestine and genitourinary tract of humans (Vandana et al., 2010). Infections in these areas lead to endovascular, central nervous system, ocular, oral bone and joint and urogenital tracts infections. It is also associated with diseases like endocarditis, bacteremia and septic arthritis (Bizzarro et al., 2011). The G. adiacens has a normal commensal relationship with most the human mucosal surfaces which allows it to affect those areas of the human body and although it has the possibilities of infecting all these areas, it rarely causes diseases (Gardenier et al., 2011).
| | Underneath the umbrella of “pesticides”, there are biologically synthesized alternatives to chemicals called microbial pesticides. This alternative uses different microorganisms to target specific pests that threaten production. The actual material used as a pesticide is naturally formed, biodegradable, highly specialized to specific pests rather than having widespread impact meaning they can be used in small quantities with large effects (EPA). Microbial pesticides are commonly used to address pest control in corn crops. Stats on the importance of corn in USA agriculture, and the threat corn poses to it (monoculture). It is under threat by the Western Corn Rootworm (CRW), which specialized just to parasitize corn monocropping productions (Cornell). A common management process to address CRW is the incorporation of microbial pesticides into corn GMO strains (background on melding process?) Bacillus thergo (Bt) is the most commonly used bacteria as a source of microbial pest control. There are multiple strains of GMO corn that contain toxins from Bt. Unfortunately, in some regions where these GMOs are overused, CRW has adapted resistance to this toxin (Cornell). This drives researchers to search for similarly effective bacteria to replace their current technology. |
| | <br> |
| | <br> |
|
| |
|
| G. adiacens bacteria are gram positive with streptococcus morphology. Sometimes it appears as cocci, coccobacilli or rod shaped cells. The cellular morphology depends on growth conditions. Their sizes range from 0.4 to 0.6 microns. G+C content of G. adiacens bacteria DNA is around 36.6 – 37.4 mol%. This bacteria is also known to be monophyletic. It is a facultative anaerobe which can survive in both aerobic and anaerobic environments with a temperature of about thirty seven (37) degrees Celsius (Collins MD et al., 2000).
| | ==Western Corn Rootworm== |
| A Granulicatella genus bacterium is a fastidious microorganism; meaning, it has a complex nutritional requirement and can only grow in a specific diet of nutrients. Most fastidious microorganisms require blood or hemoglobin, amino acids and some vitamins to grow. These types of microorganisms are known to cause infections in humans and other organisms that have blood or hemoglobin. This makes its identification difficult because a unique culture media is required for the growth of isolates of G. adiacens. The type of media used to culture these fastidious bacteria in the laboratory is known as BD Chocolate Agar. The chocolate agar is supplemented with hemoglobin (blood) and yeast concentrate. There are two common types of chocolate agars used; the BD Chocolate Agar (GC II Agar with IsoVitalex) and the BD Chocolate Agar (Blood Agar No. 2 Base). The GC II Agar with IsoVitalex base nutrients contains blood; casein; selected meat peptones as a source of nitrogen; phosphates, which helps to regulate pH; and corn starch which helps to neutralize toxic fatty acids that may be present in the agar. The Blood Agar base is sometimes used as a substitute. The Blood base agar contains blood, liver digest, Proteose peptone and yeast extract which serves as a source of nitrogen and other vitamins for the growth of the microbe that needs to be cultured. However, Granulicatella adiacens is plated on the BD Chocolate Agar (GC II Agar with IsoVitalex) to reveal growth of nutritionally variant streptococci. Most laboratories use either horse or sheep blood as a source of hemoglobin. A way of identifying these bacteria is that when plated on a BD Chocolate Agar (Blood Agar No. 2 Base), it shows a very slow growth compare to BD Chocolate Agar (GC II Agar with IsoVitalex) (Perkins et al., 2003 & “Instructions for Use – Ready-To-Use Plated Media." 2011).
| |
|
| |
|
| Scientists find this bacteria genus difficult to identify because it also appears as a gram negative bacteria sometimes and it has a range of form of shapes in which it appears which makes it uneasy to diagnose patience suffering with infections or diseases from this microbe. On normal circumstance, clinical diseases caused by G. adiacens are identified based on their phenotypic character by 16S rRNA gene sequencing. (Collins MD et al., 2000).
| | The Western Corn Rootworm (latin WCRW) is a species of Coleoptera (beetle) that specializes only in eating species of corn (latin). These beetles are ¼” long and yellow with black stripes along the sides of their elytra. They only have a single generation a year, because their life cycle is dependent on corn developmental stages (cornell source). Adult beetles lay their egg clusters below the soil surface for generation the following year, before dying themselves in the first winter frost (cornell). These eggs hatch during late May and early June into larva that are long small and white but sport brown heads with mandibles. They spend most of their larval stage feeding on the root zone of soil. They tunnel through dirt until finding corn roots to feed on. This tunneling can kill an entire root or break the tips of them, this prevents the plant from recieving enough nutrients causing it to either fall over, or greatly damage growth. The plant can be weakened in terms of stunting growth, reducing the yield amount, or even reducing the size of each ear of corn (cornell source). These larva undergo three instars of molting over a 4-6 week period (Iowa extension source). Before they pupate within the soil to undergo a compleme metamorphosis of their bodies into their adult forms. 5-10 days after pupation, CRW emerge as adults coinciding with when the corn plants flower (Iowa extension source). CRW beetles still consume during their adult stages, mainly corn pollen and corn silk, sometimes their leaves as well. Corn silk and pollen are both are needed for corn to pollinate eachother, so adults reduce their ability to reproduce (cornell source). Despite these negative impact, it is still less than the damage caused during their larval stage feeding on roots (Godfrey et al. 1993). In laying their eggs for the next generation, adult CRW are relatively stagnant species and do not tend to move fields for laying their eggs. As a result a common pest management technique for CRW is crop rotation with soy beans. Each year switching corn out for soybean plants prevents CRW from parasitizing their roots, causing CRW larva populations to drop due to the lack of their niche corn food source. Unfortunately, in some cases CRW have adapted to crop rotation schedules and have begun laying their eggs in soybean fields in anticipation of crop rotation to corn the following Spring when their offspring hatch (cornell source). This species adapts quickly due to its dependence on corn as its sole food source (Rausher et al.) As a result, other forms of pesticides are needed to be used alongside crop rotation (cornell source). One of these techniques include GMO corn strains that can be more resistant to these pests than non-GMO crops. A common GMO strain used to address CRW pest threats is Bt resistant corn. |
|
| |
|
| Taxonomy:
| | NEED TO PUT INTO CITATION & FIGURE |
| Bacteria,
| |
| Firmicutes,
| |
| Bacilli,
| |
| Lacotabacillales,
| |
| Carnobacteriaceae,
| |
| Granulicatella,
| |
| Granulicatella adiacens.
| |
| BIOS: Taxonomy (http://www.gbif.org/species/119570537)
| |
|
| |
|
| Below are links to pictures of G. adiacens culture growth of isolated colonies after two days of streaking each plate. We notice that there is a slower growth on the Blood base Agar. (Please Click on the links to view pictures)
| | ==<i>Brevibacillus laterosporus</i>== |
|
| | A number of Bacillus species have been investigated for pesticidal properties due to their formation of specialized biotoxins. Different species currently in use for biopesticides include: _____. While Bt is commonly regarded as the “most effective??” biopesticide, it has become less effective as CRW adapt to overcome its toxins (SOURCE). In recent years BL has become a bacterial species of more interest due to the speed in which it produces biotoxins, even if less powerful of a toxin than Bt produced (Ghazanchyan et al. & Oliviera et al.). |
| Sample of G. adiacens growth in BD Chocolate Agar (GC II Agar with IsoVitalex)
| |
| (http://hampc168.blog.163.com/blog/static/1697976200701910515808/)
| |
|
| |
| BD Chocolate Agar (Blood Agar No. 2 Base) showing slow rate of G. adiacens growth
| |
| (http://hampc168.blog.163.com/blog/static/1697976200701910515808/) | |
|
| |
| A case of an infected endocarditis
| |
| (http://upload.wikimedia.org/wikipedia/commons/7/73/Haemophilus_parainfluenzae_Endocarditis_PHIL_851_lores.jpg)
| |
|
| |
|
| | Brevibacillus laterosporus is rod-shaped with a gram-negative cell wall and relies on access to oxygen for metabolism. This bacteria produces a number of products including enzymes and prebiotic materials that function as an insecticides (Orlova et al.) B. lat can be sources from environmental soil, plants, and even the insects themselves (Ghazanchyan et al.). This bacteria produces endospores. On one end of each spore, this species of bacteria can be identified by its production of canoe-shaped paraposal bodies (a crystallized shell that contains endotoxins that can be used as pesticides (Ghazanchyan et al.). This bacteria has had almost an 100% effectiveness rate in the elimination of Coleopteran (beetles) pests depending on the strain, this was most effective in targeting these species during their larval stage, especially during their second instar (Favret et al.). |
|
| |
|
| | This species is of interest by biopesticide scientists due to the high rate in which it produces these paraposal bodies (pesticide containing structures) (Ghazanchyan et al.). Specialization??? These paraposal bodies can be HOW ARE THEY USED Bt resistant corn which uses toxins produced by Bacillus thergoneous to prevent parasitation. These Bt toxin BACKGROUND?? but if they are used to often CRW can become resistant to these strains. Bt is the most commonly used strain of resistant corn, and due to its over use, there are now multiple resistant strains of CRW both in Northwest Iowa and Southeast Minnesota. While these resistant strains mostly cannot move from field to field without accidental human transportation, they fortell the immunity that may be seen in other regions over-using this same resistant strain technique. As a result, new bacterial strains are being investigated to create new resistant corn GMO archetypes. Similar to crop rotation, a rotation of toxins may introduce more variety in pest control techniques that they will be less able to adapt to (cornell source). |
|
| |
|
| | ==Pathology & Experimental Use== |
| | Include some current research, with at least one figure showing data.<br> |
| | <br> |
|
| |
|
| | ==Real World Impacts== |
|
| |
|
| | ==Conclusion== |
| | Root Worms |
|
| |
|
| | | ==References== |
| | | <references /> |
| References:
| | <br><br>Authored for BIOL 238 Microbiology, taught by [https://biology.kenyon.edu/slonc/slonc.htm Joan Slonczewski], 2023, [http://www.kenyon.edu/index.xml Kenyon College] |
| | |
| Christensen JJ, Facklam RR. 2001. Granulicatella and Abiotrophia species from Human Clinical Specimens. J. Clin. Microbiol. 39(10): 3520-3523 http://jcm.asm.org/content/39/10/3520.full
| |
| | |
| Bizzarro MJ, Callan DA, Farrel PA, Dembry L-M, Gallagher PG. 2011. Granulicatella Adiacens and Early-Onset Sepsis in Neonate. Emrg Infect Dis 17(10): 1971-1973
| |
| http://jmm.sgmjournals.org/content/61/Pt_6/755.full
| |
| | |
| Vandana KE, Mukhopadhyay C, Rau NR, Ajith V, Rajath P. 2010. Native Valve Endocarditis and Femoral Emolism due to Granulicatella Adiacens: A Rare Case Report. Braz J Infect Dis 14(6) http://dx.doi.org/10.1590/S1413-86702010000600015
| |
| | |
| Perkins A, Osorio S, Serrano O, Del Ray MC, Sarria C, Domingo D, Lopez-Brea M. 2003. A Case of Endocarditis due to Granulicatella adiacens. Clinical Microbiology and Infection 9(6): 576-577 http://onlinelibrary.wiley.com/doi/10.1046/j.1469-0691.2003.00646.x/full
| |
| | |
| Gardenier JC , Hranjec T, Sawyer RG, Bonatti H. 2011. Granulicatella Adiacens Bacteremia in an Elderly Trauma Patient. Surg Infect (Larchmt) 12(3): 251-3
| |
| http://www.ncbi.nlm.nih.gov/pubmed/21524203
| |
| | |
| Collins MD, Lawson PA. 2000. The Genus Abiotrophia (Kawamura et al.) is not Monophyletic: Proposal of Granulicatella gen. nov., Granulicatella adiacens comb. nov., Granulicatella Elegans comb. nov. and Granulicatella Balaenopterae comb. nov. International Journal of Systematic and Evolutionary Microbiology. 50:365-369
| |
| http://ijs.sgmjournals.org/content/50/1/365.full.pdf
| |
| | |
| Collins MD, Lawson PA. 2000: Granulicatella adiacens (Bouvet et al., 1989) BIOS:Baceteriology Insight Orienting System in the Catalogue of Life in The Catalogue of Life Partnership: Catalogue of Life.
| |
| http://www.gbif.org/species/119570537
| |
| | |
| “Instructions for Use – Ready-To-Use Plated Media." Bd.com. Becton Dickinson, Sept. 2011. Web. 12 Dec. 2013.
| |
| http://www.bd.com/resource.aspx?IDX=8994
| |
| | |
| == Oenococcus kitaharae == | |
| | |
| Samantha Hosch
| |
| | |
| December 16,2013
| |
| | |
| Microbiology
| |
| | |
| Dr. Hahn
| |
| | |
| | |
| Lineage
| |
| | |
| • Kingdom- Bacteria
| |
| | |
| • Division- firmicutes
| |
| | |
| • Class- Bacilli
| |
| | |
| • Family- Lactobacillus
| |
| | |
| • Genius- Oenococcus
| |
| | |
| • Species- Kitaharae
| |
| | |
| | |
| | |
| No picture available
| |
| | |
| Basic
| |
| | |
| Oenococcus kitaharae is a bacteria microbe that is gram positive. It can make acid from maltose. It also helps with D-glucose fermentation. Oenococcus kitaharae is made up 42 percent guanine cytosine bonds according too Lactobacillus florum sp. nov., a fructophilic species isolated from flowers by Endo, Futagawa-Endo, Sakamoto, Kitahara, and Dicks.
| |
| | |
| It does not have the mutSL gene, which fixes some mutations and is believed by scenticsts to have not had this gene for a long time. According to the article Role of Hypermutability in the evolution of the genus Oenococcus kitaharae has a rate of 1/13 protein mutation and that most of it its mutations are random ones without any real meaning.
| |
| | |
| Oenococcus kitaharae can be grown in the lab and cultured but it does take it own time to do so, for to five days longer than most similar bacteria.
| |
| | |
| O. kitaharae can not break down anything made of organic acids but is lactic acid loving bacteria microbe. This explains why it was in shochu residue and not wine.
| |
| | |
| | |
| History
| |
| | |
| Oenococcus kitaharae was discovered in 2006. It is currently the second member of only a two-member genus. Its genus partner is Oenococcus onei, which has been renamed.
| |
| | |
| | |
| Information
| |
| | |
| The Oenococcus genus is known for their ability to be involved with fermentation for this reason and the fact that it is present in wine the genus is studied often. However onei and kitaharae have different living environments but can have crossovers, this has been shown through PCR reactions from wine samples.
| |
| Some sources show that O. kitaharae can cause fermentation in some of O. onei environments well other show that it does just want it needs to stay a live.
| |
| No matter what there is no disagreement on the fact the fact that they can be found together.
| |
| | |
| Oenococcus kitaharae is much able to survive in difficult environment. Apparently, It has more DNA in the Oenococcus kitaharae. The extra pieces of DNA resemble that of a virus according an article titled Comparative Genomics of Oenococcus kitaharae. It is found in Japan in several things including flowers.
| |
| | |
| | |
|
| |
| | |
| Sources
| |
| | |
| Borneman, A. R., McCarthy, J. M., Chambers, P. J., & Bartowsky, E. J. (2012). Functional divergence in the genus oenococcus as predicted by genome sequencing of the newly- described species, oenococcus kitaharae. PLoS One, 7(1), e29626. doi: 10.137
| |
| | |
| Endo, A., Futagawa-Endo, Y., Sakamoto, M., Kitahara, M., & Dicks, D. M. T. (2010). Lactobacillus florum sp. nov., a fructophilic species isolated from flowers. International Journal of Systematic and Evolutionary Microbiology, 60, 2478–2482. doi: 10.1099/ijs.0.019067-0
| |
| | |
| Endo, A., & Okada, S. (2006). Oenococcus kitaharae sp. nov., a non-acidophilic and non- malolactic-fermenting oenococcus isolated from a composting distilled shochu residue. International Journal of Systematic and Evolutionary Microbiology, (56), 2345-2348. doi: 10.1099/ijs.0.64288-0
| |
| Gonzalez-Arenzana, L., Lopez, R., Santamaría, P., & Lopez-Alfaro, I. (2013). Dynamics of lactic acid bacteria populations in rioja wines by pcr-dgge comparison with culture-dependent methods . Appl Microbial Biotechnol, (97), 6931-6941. doi: 10.1007/s00253-013-4974-y
| |
| Marcobal, A. M., Sela , D. A., Wolf, Y. I., Makarova, K. S., & Mills, D. A. (2008). Role of hypermutability in the evolution of the genus oenococcus. Journal Of Bacteriology, 190(2), 564-570. doi: 10.1128/JB.01457-07
| |
| | |
| Michlmayr, H., Schümann, C., Wurbs, P., arreira Braz da Silva, N. M., Rogl, V., Kulbe, K. D., & del Hierro, A. M. (2010). A β-glucosidase from oenococcus oeni atcc baa-1163 with potential for aroma release in wine: Cloning and expression in e. coli. World J Microbiol Biotechnol, 26(7), 1281-1289. doi: 10.1007/s11274-009-0299-5
| |
| | |
| == Pseudomonas fluorescens ==
| |
| | |
| Pseudomonas fluorescens Microbe Wiki
| |
| | |
| Classification
| |
| Kingdom: Bacteria
| |
| Phylum: Proteobacteria
| |
| Class: Gammaproteobacteria
| |
| Order: Pseudomonadales
| |
| Family: Pseudomonadaceae
| |
| Genus: Pseudomonas
| |
| Species: P. fluorescens
| |
| | |
| Habitat Information:
| |
| The soil organism was collected in the front yard of an Austin, TX home on January 26, 2018.
| |
| Soil was a little moist
| |
| Picked up on a day that had 83% humidity
| |
| Zero rainfall
| |
| Calm wind
| |
| 51℉ air temperature.
| |
| | |
| Pseudomonas fluorescens is mainly found in plants, soil, and water surfaces.
| |
| | |
| Description and Significance:
| |
| Pseudomonas fluorescens are gram-negative bacilli shaped bacteria. It grows best in temperatures that are 25-30℃. Certain strains of Pseudomonas fluorescens have been found to help stop plant disease by protecting the root and seed from fungal infection[REF]. Other strains contribute to plant growth. Due to P. fluorescens having different flagella it has different strains which cause it to be in different environments including the bloodstream. [REF]
| |
| | |
| Cell Structure, Metabolism, and Life Cycle:
| |
| Cell Structure
| |
| | |
| P. fluorescens are small-to-medium sized Gram-negative, rod-shaped bacilli. They are often found with multiple flagella in a lophotrichous arrangement. These many flagella, along with its ability to generate a biofilm, make P. fluorescens a great colonizer on various different surfaces and in different hosts and able to easily adapt to its environment[REF]. One particularly prominent role of this biofilm is to serve as a protective agents to plants against parasitic fungi. Less is known about how P. fluorescens’ structure allows it to bind to mammalian cells, however it has been known to adhere to red blood cells in humans, which is one reason it is believed that, when found as a pathogenic agent in humans (which is very rare), it is almost always in the bloodstream. This organism follows a similar life cycle pattern found with other biofilm generating species, as discussed in “Life Cycle” [REF].
| |
| | |
| Metabolism
| |
| P. fluorescens is well-known for having an extensive variety of metabolic capabilities, which allows it to live in so many different environments such as on the surfaces of plants, in soil, in the rhizosphere, and even in the bloodstream of humans and other animals[REF].
| |
| | |
| P. fluorescens is a obligate aerobe, however, it has a unique ability to use nitrate (NO3) instead of atmospheric oxygen (O2) as its final electron acceptor in the Electron Transport Chain [REF]
| |
| | |
| A unique metabolic feature of P. fluorescens is that it secretes a fluorescent pigment, pyoverdine, which imparts fluorescent properties to the organism under UV light, which is what led to its name. Pyoverdine is a high-affinity iron-chelating molecule that is essential for the organism’s acquisition of iron from the environment and used for bacterial growth. [REF]
| |
| See more in “Physiology” for biochemical tests conducted in class.
| |
| | |
| Life Cycle
| |
| P. fluorescens follows a typical “biofilm” life cycle in that generally proceeds as follows:
| |
| Attachment: planktonic cells adhere to a surface and become sessile
| |
| Growth: cells exude exoenzymes and proteins to create a protective biofilm in which to flourish and grow.
| |
| Detachment: individual cells or clusters of cells will detach from the biofilm in order to move and colonize new surfaces/hosts
| |
| | |
| Genome Structure
| |
| P. fluorescens’ genome is composed of a single, circular chromosome with a median length of 6,300,000 base pairs. Guanine and Cytosine make up 60.3% of the nucleotides found in its DNA (its G/C ratio). [REF]
| |
| | |
| | |
| | |
| | |
| | |
| Physiology and Pathogenesis:
| |
| Physiology
| |
| | |
| Gelatin Hydrolysis: Negative
| |
| DNA Hydrolysis: Negative
| |
| Lipid Hydrolysis: Positive
| |
| Phenol Red Broth: No fermentation
| |
| Starch Hydrolysis: Negative
| |
| Casein Hydrolysis: Positive
| |
| Methyl Red: Negative
| |
| Voges-Proskauer: Negative
| |
| Citrate: Positive
| |
| SIM: Negative
| |
| Nitrate Reduction: Positive
| |
| Urea Hydrolysis: Negative
| |
| Triple Sugar Iron: No fermentation, does not reduce sulfur
| |
| Decarboxylation: Arginine is positive, lysine and ornithine are negative
| |
| Phenylalanine: Negative
| |
| Oxidase: Positive
| |
| EMB Agar: Positive
| |
| HE Agar: Negative
| |
| Catalase: Positive
| |
| Blood Agar: Positive
| |
| Mannitol Salts Agar: Negative
| |
| PEA Agar: Negative
| |
| | |
| Bile Esculin: Negative
| |
| 6.5% Salt Tolerance: Negative
| |
| Kirby-Bauer Antimicrobial Susceptibility Test for disinfectants:
| |
| Kirby-Bauer Antimicrobial Susceptibility Tests for antibiotics: sensitive to several antibiotics [REF]
| |
| | |
| Pathophysiology
| |
| Although P. fluorescens itself is largely considered non-pathogenic, it contains a number of metabolic abilities to allow it to thrive in mammalian hosts, including, but not limited to:
| |
| Production of bioactive secondary metabolites
| |
| P. fluorescens produces a long list of secondary metabolites that allow it to successfully compete with other, similar organisms, such as phenazine, hydrogen cyanide, 2,4-diacetylphloroglucinol (DAPG), rhizoxin, and pyoluteorin. [REF]
| |
| Production of biofilms
| |
| As aforementioned, one of the key structural components of P. fluorescens is its ability to produce biofilms.
| |
| Type III secretions
| |
| Type III secretion systems (T3SSs) are molecular, needle-like complexes that inject cellular products into the cells of its host/surface, known as effectors. The most common T3SS in P. fluorescens is the Hrp1 family[REF]. These “hypersensitive response” secretion systems trigger a hypersensitive response in resistant plants, but leads to infection in vulnerable plants. Less is known about T3SSs involved in this organism’s infections in mammals, but different strains have been found to adhere to human Red Blood Cells, as well as human glial cells in culture. [REF]
| |
| | |
| References
| |
| Ramette A, Moënne-Loccoz Y, Défago G, Prevalence of fluorescent pseudomonads producing antifungal phloroglucinols and/or hydrogen cyanide in soils naturally suppressive or conducive to tobacco black root rot. FEMS Microbiol Ecol. 2003 May 1; 44(1):35-43.
| |
| Gibaud M, Martin-Dupont P, Dominguez M, Laurentjoye P, Chassaing B, Leng B. Pseudomonas fluorescens septicemia following transfusion of contaminated blood.
| |
| Presse Med. 1984 Nov 24; 13(42):2583-4.
| |
| Scales BS, Dickson RP, LiPuma JJ, Huffnagle GB. 2014. Microbiology, genomics, and clinical significance of the Pseudomonas fluorescens species complex, an unappreciated colonizer of humans. Clin Microbiol Rev 27:927–948. doi:10.1128/CMR.00044-14.
| |
| Hernández-Salmerón JE, et al. Draft Genome Sequence of the Biocontrol and Plant Growth-Promoting Rhizobacterium Pseudomonas fluorescens strain UM270. Stand Genomic Sci 2016
| |
| Ghiglione JF, Gourbiere F, Potier P, Philippot L, Lensi R. Role of respiratory nitrate reductase in ability of Pseudomonas fluorescens YT101 to colonize the rhizosphere of maize. Appl Environ Microbiol. 2000;66(9):4012–4016. Doi: 10.1128/AEM.66.9.4012-4016.2000
| |
| Hohnadel D, Meyer JM. Specificity of pyoverdine-mediated iron uptake among fluorescent Pseudomonas strains. J Bacteriol. 1988 Oct; 170(10):4865-73.
| |
| Baum MM, Kainović A, O'Keeffe T, Pandita R, McDonald K, Wu S, Webster P. Characterization of structures in biofilms formed by a Pseudomonas fluorescens isolated from soil. BMC Microbiol. 2009 May 21; 9():103
| |
| Adebusuyi AA, Foght JM. An alternative physiological role for the EmhABC efflux pump in Pseudomonas fluorescens cLP6a. BMC Microbiol. 2011;11:252. doi: 10.1186/1471-2180-11-252. [Online.]
| |
| Preston GM, Bertrand N, Rainey PB. Type III secretion in plant growth-promoting Pseudomonas fluorescens SBW25.
| |
| Mol Microbiol. 2001 Sep; 41(5):999-1014.
| |
| Chapalain A, Rossignol G, Lesouhaitier O, Merieau A, Gruffaz C, Guerillon J, Meyer JM, Orange N, Feuilloley MG. Comparative study of 7 fluorescent pseudomonad clinical isolates.Can J Microbiol. 2008 Jan; 54(1):19-27.
| |
Brevibacillus laterosporus, a bacterial biological control agent of Western Corn Rootworm.
Background
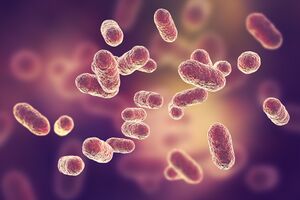
This illustration depicts a three-dimensional (3D), computer-generated image, of a group of Gram-positive, Streptococcus agalactiae (group B Streptococcus) bacteria. The photo credit for this image belongs to Alissa Eckert, who is a medical illustrator at the
CDC.
By Katya Naphtali
Underneath the umbrella of “pesticides”, there are biologically synthesized alternatives to chemicals called microbial pesticides. This alternative uses different microorganisms to target specific pests that threaten production. The actual material used as a pesticide is naturally formed, biodegradable, highly specialized to specific pests rather than having widespread impact meaning they can be used in small quantities with large effects (EPA). Microbial pesticides are commonly used to address pest control in corn crops. Stats on the importance of corn in USA agriculture, and the threat corn poses to it (monoculture). It is under threat by the Western Corn Rootworm (CRW), which specialized just to parasitize corn monocropping productions (Cornell). A common management process to address CRW is the incorporation of microbial pesticides into corn GMO strains (background on melding process?) Bacillus thergo (Bt) is the most commonly used bacteria as a source of microbial pest control. There are multiple strains of GMO corn that contain toxins from Bt. Unfortunately, in some regions where these GMOs are overused, CRW has adapted resistance to this toxin (Cornell). This drives researchers to search for similarly effective bacteria to replace their current technology.
Western Corn Rootworm
The Western Corn Rootworm (latin WCRW) is a species of Coleoptera (beetle) that specializes only in eating species of corn (latin). These beetles are ¼” long and yellow with black stripes along the sides of their elytra. They only have a single generation a year, because their life cycle is dependent on corn developmental stages (cornell source). Adult beetles lay their egg clusters below the soil surface for generation the following year, before dying themselves in the first winter frost (cornell). These eggs hatch during late May and early June into larva that are long small and white but sport brown heads with mandibles. They spend most of their larval stage feeding on the root zone of soil. They tunnel through dirt until finding corn roots to feed on. This tunneling can kill an entire root or break the tips of them, this prevents the plant from recieving enough nutrients causing it to either fall over, or greatly damage growth. The plant can be weakened in terms of stunting growth, reducing the yield amount, or even reducing the size of each ear of corn (cornell source). These larva undergo three instars of molting over a 4-6 week period (Iowa extension source). Before they pupate within the soil to undergo a compleme metamorphosis of their bodies into their adult forms. 5-10 days after pupation, CRW emerge as adults coinciding with when the corn plants flower (Iowa extension source). CRW beetles still consume during their adult stages, mainly corn pollen and corn silk, sometimes their leaves as well. Corn silk and pollen are both are needed for corn to pollinate eachother, so adults reduce their ability to reproduce (cornell source). Despite these negative impact, it is still less than the damage caused during their larval stage feeding on roots (Godfrey et al. 1993). In laying their eggs for the next generation, adult CRW are relatively stagnant species and do not tend to move fields for laying their eggs. As a result a common pest management technique for CRW is crop rotation with soy beans. Each year switching corn out for soybean plants prevents CRW from parasitizing their roots, causing CRW larva populations to drop due to the lack of their niche corn food source. Unfortunately, in some cases CRW have adapted to crop rotation schedules and have begun laying their eggs in soybean fields in anticipation of crop rotation to corn the following Spring when their offspring hatch (cornell source). This species adapts quickly due to its dependence on corn as its sole food source (Rausher et al.) As a result, other forms of pesticides are needed to be used alongside crop rotation (cornell source). One of these techniques include GMO corn strains that can be more resistant to these pests than non-GMO crops. A common GMO strain used to address CRW pest threats is Bt resistant corn.
NEED TO PUT INTO CITATION & FIGURE
Brevibacillus laterosporus
A number of Bacillus species have been investigated for pesticidal properties due to their formation of specialized biotoxins. Different species currently in use for biopesticides include: _____. While Bt is commonly regarded as the “most effective??” biopesticide, it has become less effective as CRW adapt to overcome its toxins (SOURCE). In recent years BL has become a bacterial species of more interest due to the speed in which it produces biotoxins, even if less powerful of a toxin than Bt produced (Ghazanchyan et al. & Oliviera et al.).
Brevibacillus laterosporus is rod-shaped with a gram-negative cell wall and relies on access to oxygen for metabolism. This bacteria produces a number of products including enzymes and prebiotic materials that function as an insecticides (Orlova et al.) B. lat can be sources from environmental soil, plants, and even the insects themselves (Ghazanchyan et al.). This bacteria produces endospores. On one end of each spore, this species of bacteria can be identified by its production of canoe-shaped paraposal bodies (a crystallized shell that contains endotoxins that can be used as pesticides (Ghazanchyan et al.). This bacteria has had almost an 100% effectiveness rate in the elimination of Coleopteran (beetles) pests depending on the strain, this was most effective in targeting these species during their larval stage, especially during their second instar (Favret et al.).
This species is of interest by biopesticide scientists due to the high rate in which it produces these paraposal bodies (pesticide containing structures) (Ghazanchyan et al.). Specialization??? These paraposal bodies can be HOW ARE THEY USED Bt resistant corn which uses toxins produced by Bacillus thergoneous to prevent parasitation. These Bt toxin BACKGROUND?? but if they are used to often CRW can become resistant to these strains. Bt is the most commonly used strain of resistant corn, and due to its over use, there are now multiple resistant strains of CRW both in Northwest Iowa and Southeast Minnesota. While these resistant strains mostly cannot move from field to field without accidental human transportation, they fortell the immunity that may be seen in other regions over-using this same resistant strain technique. As a result, new bacterial strains are being investigated to create new resistant corn GMO archetypes. Similar to crop rotation, a rotation of toxins may introduce more variety in pest control techniques that they will be less able to adapt to (cornell source).
Pathology & Experimental Use
Include some current research, with at least one figure showing data.
Real World Impacts
Conclusion
Root Worms
References
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2023, Kenyon College

