Chlamydophila pneumoniae in Atherosclerosis
Chlamydophila pneumoniae (C. pneumoniae) is a bacteria most widely known as a human respiratory pathogen but has recently been studied for its role in atherosclerosis: a chronic systemic arterial inflammatory disease caused by the buildup of fatty materials. The involvement of C. pneumoniae in atherosclerosis has been investigated by seroepidemiological studies, by histopathological studies, and in animal models. Mechanisms by which C. pneumoniae may alter properties of the cardiovascular wall may include the bacteria's role in foam cell formation (early atherosclerotic lesions) and its ability to induce growth factors and pro-inflammatory cytokines. Studies on possible vaccinations against these bacteria are underway.
Background
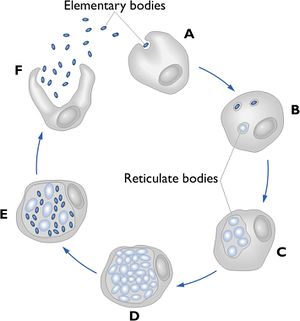
The genus Chlamydia is composed of small, gram-negative intracellular bacteria that depend on their host for growth and survival. C. pneumoniae undergoes a unique biphasic developmental cycle consisting of two morphologically and functionally distinct forms: the elementary body (EB) and the reticulate body (RB). The EB is sometimes called the survival form because it has the ability to survive outside of human cells (Figure 2F). Once conditions are suitable, attachment occurs (Figure 2A) and RBs are formed inside the host where they actively divide (Figure 2B, C, D). The RB replicates and forms a microcolony inside the cell and then differentiates again back into its EB form (Figure 2E), which is then released from the infected cell to begin the next cycle. Under some conditions, the RBs do not differentiate back into EBs, but form ‘inclusion bodies’ (Figure 1, part 3) that do not replicate and allow the bacterium to maintain a chronic latent infection (Park 2010). Figure 2 shows this biphasic life cycle with both the EBs and RBs. Unlike other human chlamydial pathogens such as C. trachomatis, C. pneumoniae can infect and survive in a wide variety of host cell types such as resident macrophages, lung epithelium, arterial smooth muscle cells, circulating monocytes, and vascular endothelium (Belland 2004).
Recently, C. pneumoniae has been studied for its implications in the onset or progression of several nonpulmonary diseases such as atherosclerosis. This research development was sparked when investigators began looking at other bacteria such as Helicobacter pylori in the early 1990s (Campbell 1998). These studies concluded that the connection between infection and atherosclerosis is much stronger for C. pneumoniae than for Helicobacter pylori or any other bacteria (Campbell 1998). Interestingly, a large number of studies reported an association between C. pneumoniae and symptoms of atherosclerosis such as carotid artery stenosis (narrowing or constriction of the inner surface of the carotid artery), coronary artery disease (CAD) (plaque build-up inside coronary arteries), and lower extremities artery obstruction (arterial plaque build-up in hips, legs, and calves) (Brykczynski 2012).
Atherosclerosis
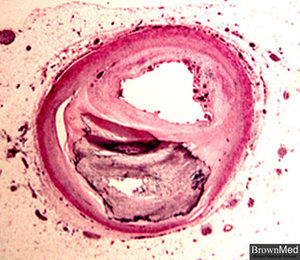
Cardiovascular disease (CVD) is one of the leading health problems in both developed and developing countries (Watson 2008). Clinical manifestations of CVD include acute coronary syndrome (any symptoms attributed to obstruction of coronary arteries), stroke (rapid loss of brain function due to disturbance of blood supply to the brain), and angina pectoris (chest pain due to obstruction of coronary arteries). The main pathological process leading to these cardiovascular events is atherosclerosis (the accumulation of fatty materials leading to artery wall thickening) (Watson 2008). The lesions of atherosclerosis occur primarily in elastic (large) and muscular (small) arteries and are composed of three distinct components: 1) the atheroma (yellowish material at the center composed of macrophages); 2) the underlying cholesterol crystals and; 3) the calcification at the outer base of older or more advanced lesions (Figure 3). (Ross 1999). In Figure 3, calcium deposits can be seen to the left of the plaque. The entire plaque is covered by a fibromuscular cap.
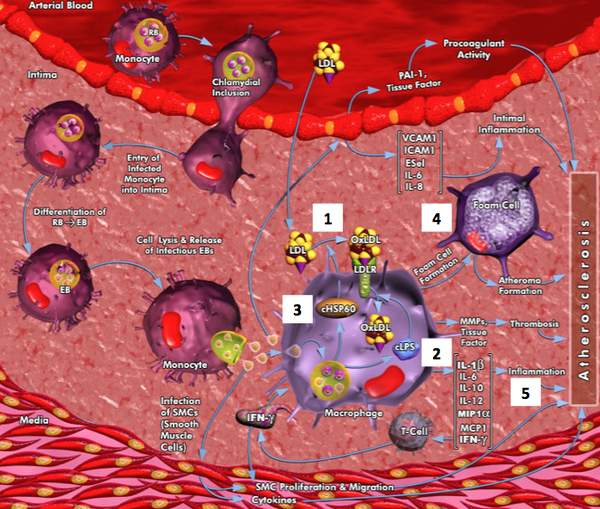
Atherosclerosis is a disease that usually develops several years before symptoms manifest themselves (Ross 1999). When low-density lipoprotein (LDL) deposits become oxidized (oxLDL) (Figure 4, #1) within the vascular wall of the heart, it stimulates the migration of monocytes into the subendothelial compartment (Watson 2008). These cells absorb oxLDL via scavenger receptors (SRs) and become activated foam-cell macrophages (Figure 4, #4), also known as ‘fatty streaks’ (Figure 4) (Watson 2008). The interactions between inflammatory macrophages and lymphocytes and repair responses continue over many years increasing the volume of the atherosclerotic plaque. These plaques consist of a fibrous cap shielding the vessel lumen (open space of a blood vessel) from the macrophage-rich lipid core. As inflammatory activity (Figure 4, #5) continues within the plaques, it can lead to weakening of the cap, increasing the probability that it will rupture, releasing lipid material. This material activates platelets, and can result in the formation of a thrombus (blood clot) or eventually angina (cardiac chest pain) or myocardial infarction (heart attack). Figure 4 refers to this mechanism and different components of the figures (#1-5) will be discussed later.
Association of C. pneumoniae with atherosclerosis
There is evidence to suggest that C. pneumoniae could play a role in all stages of atherosclerosis, from the initial lesion to plaque rupture (Kalayoglu 2000). The strongest evidence for an association of C. pneumoniae with atherosclerosis has been shown by i) sero-epidemiological studies; ii) histopathological studies, and; iii) animal models.
Sero-epidemiological Studies
Sero-epidemiological studies analyze the levels of a specific antibody titre in a group of individuals. In the following sero-epidemiological studies, the number of specific C. pneumoniae antibodies were compared between atherosclerotic patients and healthy individuals.
The first report of a possible association between C. pneumoniae infection and atherosclerosis arose in 1988 from a small cross-sectional study in Helsinki, which showed that patients with chronic stable CAD or acute myocardial infarction (AMI) were significantly more likely to have increased C. pneumoniae antibodies than controls (50-60% and 7-12%, respectively) (Saikku 1988). This prompted further studies to investigate the association between the disease and the organism. Follow-up studies confirmed the Helsinki findings by showing an association between elevated antibody titres and angiographically verified coronary heart disease (a test that uses dyes and x-rays to show the insides of the coronary arteries) (Thom 1991). However, these cross-sectional studies are poor indicators of causality since both factors are observed at the same time. In addition, most of the sero-epidemiological studies performed at this time involved elderly populations, which is not a good measure due to the high prevalence of C. pneumoniae seropositivity in these age groups (Campbell 1998). Further, older populations are experiencing later stages of the disease, making it hard to determine a potential causal role for C. pneumoniae early in the atherosclerotic process (Campbell 1998). Therefore, histopathological studies and animal studies are typically more credible.
Histopathological Studies
Histopathological studies look at the microscopic anatomy of cells and tissues. The following histopathological studies examined cells and tissues by sectioning and staining, under a light or electron microscope.
Evidence for the presence of C. pneumoniae in atherosclerotic lesions has emerged from more than 40 studies, conducted by several different groups of investigators. Data collected from 43 studies, published before October 2002, indicate a high prevalence of C. pneumoniae (46% of 1852 specimens) in atheromatous tissue but not (<1% of more than 239 specimens) in healthy arteries (Kalayoglu 2002). These studies involved immunohistochemistry (IHC), electron microscopy (EM), polymerase chain reaction (PCR) and cell culturing.
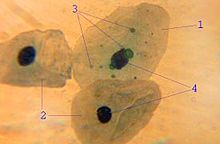
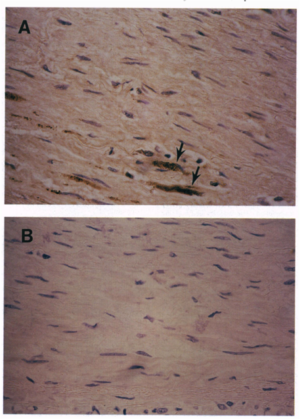
Shor et al. (1992) were the first to show that C. pneumoniae was present in confirmed atherosclerotic lesions. Using transmission electron microscopy (TEM), researchers were able to detect C. pneumoniae in macrophage foam cells (Figure 5A) (Shor 1992). Macrophage foam cells are lipid-loaded macrophages that are surrounded by a fatty substance, usually cholesterol. They are formed when the body sends macrophages to the location of a fatty deposit on the blood vessel wall. One of the characteristics of atherosclerosis is the presence of these foam cells, as seen in Figure 5. The fact that C. pneumonia has been found in these fatty macrophages is a strong indicator of the bacteria’s role in the disease.
In a subsequent study aimed at looking at younger populations (Kuo 1995), C. pneumoniae was found in atherosclerotic lesions in autopsy tissue taken from individuals between the ages of 15 and 34. The bacteria was found in the foam cells localized in the deep layer of the atherosclerotic plaque (Kuo 1995). Investigators were able to conclude that C. pneumoniae can be found in a high proportion of atherosclerotic plaque lesions in the coronary arteries of young individuals, which was different than previous histopathological studies and sero-epidemiological studies that had looked primarily at elderly populations (Kuo 1995). A second notable finding was that in tissue without evidence of atherosclerosis, no C. pneumonia was found using the same techniques (Kuo 1995). This finding is significant because it begins to explain causality rather than correlation. Findings of C. pneumonia in younger atherosclerotic tissues is more powerful than in elderly tissue due to the already high prevalence of the bacteria in so much of the population. Therefore, it being found in atherosclerotic tissue and not in healthy tissue of young patients predicts a causative role.
While the presence of the bacteria in infected tissue is notably significant, additional studies showed that C. pneumoniae is markedly absent from non-diseased tissue or tissue unrelated to the site of disease (lung, liver, spleen, bone marrow, and lymph node tissue) (Jackson 1997). C. pneumoniae was detected in none of the normal appearing coronary arteries, 33% of arteries with intimal thickening or early plaques, and 38% of arteries with advanced plaque (Jackson 1997). Of the 21 samples that tested positive for C. pneumoniae, 11 only tested positive for cardiovascular tissue, 6 tested positive for both cardvioascular and noncardviovascular positive tissue, and 3 tested positive for only noncardiovascular positive tissue (Jackson 1997). This investigation showed that C. pneumoniae is preferentially localized to cardiovascular tissue, adding evidence in support of the hypothesis that C. pneumoniae may play a role in the pathogenesis of atherosclerosis.
Animal Model Studies
Most investigators using animal models have introduced the pathogen in the respiratory tract (to simulate the portal of entry in human infection), and then examined vascular tissue for atherosclerosis and the presence of the pathogen. De Kruif and his colleagues found that C. pneumonia disseminates to vasculature and multiple organs. This presence of the bacteria in the lungs and spleen, after intranasal inoculation, provided the first experimental evidence that C. pneumonia is able to disseminate via the blood stream throughout the body (De Kruif 2005). These results suggest that C. pneumoniae exhibits tropism for vascular tissue and may accelerate the development of disease in hyperlipidemic animals.
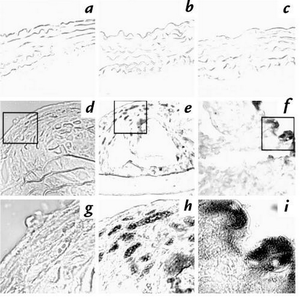
Animal models tested involved genetically-induced hyperlipidaemic mice, diet-induced hyperlipidaemic mice and cholesterol fed New Zealand white rabbits. In all three of these models, repeated C. pneumonia infection was shown to accelerate plaque development. Figure 6 comes from a study done with diet-induced hyperlipidaemic mice. In parts f and i where the mice were treated with a high-cholesterol diet plus infection with C. pneumoniae, the chlamydial LPS antigen was detected in the aorta (Hu 1999). In contrast, the LPS antigen was not detected in mice that did not receive chlamydial infection (Fig 6, d and g) (Hu 1999). Interestingly, no obvious chlamydial antigen was detected in aortic sections from mice fed a normal mouse chow and infected with C. pneumonia (Fig 6c) (Hu 1999). Images taken at a higher power objective lens (63x) (Fig 6, g-i), further revealed chlamydial inclusion body-like structures (Fig 6i) (Hu 1999). This suggests that C. pneumonia is a co-risk factor with hyperlipidaemia and that atherogenic effects of C. pneumonia are contingent on the body’s response to hyperlipidaemia (Watson 2008). In all of these animal models, C. pneumonia was identified in foam cells or at sites of inflammation (Hu 1999).
Interestingly, other pathogens such as Mycoplasma pneumonia, Helicobacter pylori and Chlamydia trachomatis have been found to not induce atheromatous changes (Sessa 2009). Taken together, it can be deduced that C. pneumoniae has an affinity for the vasculature where it can induce inflammation, and can initiate or promote lesion development in mice with atherosclerosis (Kalayoglu 2002).
Pathogenesis
Studies looking to identify mechanisms by which C. pneumoniae may alter properties of the vessel wall are ongoing. Data emerging from these in vitro experiments focus on the host cell response to infection and have identified several pathways that are activated in atherogenesis. For example, C. pneumoniae lipopolysaccharide (Figure 4, #2) has been shown in vitro to enhance foam cell formation in macrophages (Figure 4, #4) exposed to oxidized low-density lipoprotein (LDL) (Figure 4, #1) (Kalayoglu 1999, Kalayoglu 2000). Another C. pneumoniae component, chlamydial heat-shock protein 60 (Figure 4, #3), has been shown to promote the oxidation of LDL to its proatherogenic form and stimulate the synthesis of matrix metalloproteinases in macrophages (Kol 1998).
In addition to the formation of foam cells and oxidation of LDL, studies have been done on the role of C. pneumoniae and its activation of cytokines and growth factors. The organism has been shown to induce the production of proinflammatory cytokines, providing yet another mechanism for which atherosclerotic plaque could develop.
Chlamydial lipopolysaccharide (cLPS) in Foam Cell Formation
The most well-studied mechanism is C. pneumoniae's ability to modulate the interactions between macrophages and lipoproteins to produce foam cells. The formation of foam cells and oxidation of LDL are the beginnings of early atherosclerotic lesions and results from the uptake of oxLDL into macrophages (Kalayoglu 1999). Native unmodified LDL does not normally contribute to foam cell formation due to strict control of LDL receptor (LDLr) expression (Kalayoglu 2000). However, C. pneumoniae induces macrophages (unknown how) to take up increased amounts of native LDL and become foam cells (Watson 2008).
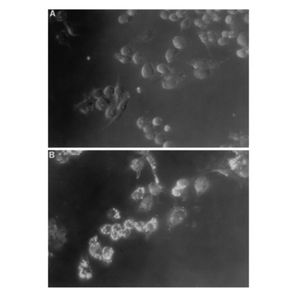
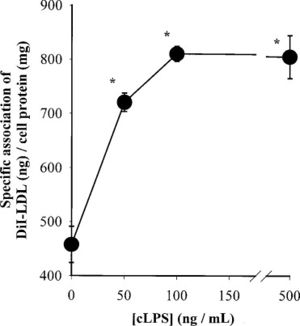
To test whether purified chlamydial lipopolysaccharide (cLPS) could induce foam cell formation, Kalayoglu and Byrne exposed macrophages to cLPS at various doses and incubated in the presence of LDL (Kalayoglu 1998). The results indicated that cLPS was sufficient to induce cholesteryl ester accumulation and foam cell formation (Kalayoglu 1998). In addition, using light and dark-field microscopy, the scientists were able to show that macrophages exposed to C. pneumoniae accumulated cytoplasmic cholesteryl ester droplets in the presence of LDL (Figure 7) (Kalayoglu 1998). Therefore, when in the presence of LDL, C. pneumoniae can induce human macrophage foam cell formation. Additional studies showed that cLPS was sufficient to induce DiI-LDL (labeled LDL) uptake into macrophages (Figure 8), suggesting a possible mechanism for how the foam cells form (Kalayoglu 1999). These were critical findings because it showed that, i) cLPS induces foam cell formation, ii) LDL and C. pneumoniae cause foam cell formation, which ultimately led to, iii) cLPS inducing LDL uptake into macrophages.
Chlamydial Heat Shock Protein 60 (Chsp60) in Oxidation of LDL
It has been hypothesized that Chlamydial HSP 60 (chsp60) is another component in C. pneumoniae -induced monocyte LDL oxidation. Before studies were done, it was known that chlamydiae produce large amounts of heat shock protein 60 (HSP 60) during chronic, persistent infections, and C. pneumoniae localizes predominantly within plaque macrophages (Huittinen 2002). Therefore the question was, what role, if any, does chsp60 play?
It has been found that Chlamydial HSP 60 frequently colocalizes with human HSP 60 in plaque macrophages in human atherosclerotic lesions (Kol 1998). The homology between human and chlamydial HSP 60 suggests the possibility of antigenic mimcry, meaning that it is possible that chronic infection with C. pneumoniae, through the expression of HSP 60, may provoke an autoimmune reaction against human HSP 60. In addition, chsp60 and human HSP 60, together, were found to induce tumor necrosis factor-α (TNF-α) and matrix-degrading metalloproteinase (MMP) production by macrophages (Kol 1999). These events may contribute to plaque weakening and subsequent rupture (Kol 1998). Induction of such macrophage functions provides a potential mechanism by which chlamydial infections may promote atherogenesis and lead to ischemic events.
Cytokines and Growth Factors
Another mechanism by which C. pneumoniae could contribute to atherosclerosis is through the activation of cytokines (proteins that regulate inflammatory responses). C. pneumoniae activates several host cell signaling pathways with downstream immunological and regulatory function effects (Coombes 2001). Interestingly, signal transduction cascades involving protein tyrosine kinases are induced within five minutes of C. pneumoniae binding to host endothelial cells (Krull 1999).
cDNA microarrays were used to study the transcriptional response of endothelial cells to infection with C. pneumoniae (Coombes 2001). Endothelial cells were chosen because they are key targets for C. pneumoniae infection given that they are, i) very important in regulating the dynamics of the vessel wall and, ii) that in vitro data has suggested that infected macrophages can transmit the C. pneumoniae infection directly to endothelial cells (Coombes 2001). Genes coding for cytokines (interleukin-1), chemokines (monocyte chemotactic protein 1 and interleukin-8) and cellular growth factors (heparin-binding epidermal-like growth actor, basic fibroblast growth factor, and platelet-derived growth factor B chain) were the most up-regulated (Table1) (Coombes 2001). The up-regulation of these proinflammatory factors such as cytokines, chemokines, growth factors, and cellular receptors all support the association between C. pneumoniae and atherosclerosis.
A second key conclusion from this study was that infection of endothelial cells with C. pneumoniae induced expression of relatively few genes (20 out of 268) (Coombes 2001). This suggests that the endothelial response to chlamydial infection is not a generalized inflammatory response (Coombes 2001). Several more studies are needed to map out the entire response with all of the implications.

Vaccine Efforts
Due to the high prevalence of C. pneumoniae, the discovery of an effective vaccine would have large-scale benefits for human health. However, while considerable progress towards developing a vaccine has been made in the past ten years, none have offered long-lasting immunity in humans. The key challenge has been finding a vaccine to confer a stronger immunity against natural infection, since reinfection is so common among humans (Watson 2008).
Some studies have tested the effect of basic antibiotics in treating experimental atherogenesis in Chlamydia-infected mice and rabbits. It was reported that azithromycin prevented accelerated atherosclerosis in hyperlipidemic rabbits infected with C. pneumonia but did not eradicate the bacteria (Rothstein 2001).
Studies since have focused on attacking cytoplasmic proteins or outer membrane proteins characteristic to the chlamydia genus (Penttila 2000, Thorpe 2007). Penttila et. al. (2000) focused on the major outer membrane protein (MOMP), cysteine-ich outer membrane protein 2 (Omp2) and the heat shock protein 60 (Hsp60). While the immunization with pmomp or phshp60 showed reduced IFU counts in the lungs of the mice, it did not offer protection from pneumonia and did not reduce the severity of histologically assessed pneumoniae (Penttila 2000).
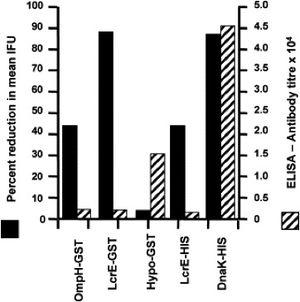
In 2007, Thorpe et. al. reported a study using a recombinant protein vaccine that induced an immune response in mice that was able to eradicate the infection. The investigators looked at surface expressed or secreted proteins found in the Chlamydial family (Thorpe 2007). One particular antigen, called LcrE, induced CD4+ and CD8+ T cell activation, Type I cytokine secretion and neutralizing antibodies (Figure 9) (Thorpe 2007). Antibodies against OmpH and LcrE significantly reduced antibody titres (Figure 9), while antibodies against Hypo did not. While the infection was eradicated, it is difficult to say it will do the same in humans due to the short lifespan of the infection in mice. Therefore, further studies are needed to test this vaccine.
Conclusion
C. pneumoniae has been shown to play a role in atherosclerosis through sero-epidemiological studies, histopathological studies, and animal model studies. Sero-epidemiogical studies have shown increased antibody titres in patients with coronary artery disease and acute myocardial infarction. Histopathological studies have been able to show C. pneumoniae present in atherosclerotic lesions through PCR, immunocytochemical staining, and electron microscopy. These studies have also been crucial for showing the absence of the organism in non-atherosclerotic tissue and tissues at other sites in the body. Animal studies done with hyperlipidaemic and nonhyperlipidaemic mice, as well as with New Zealand white rabbits, have shown that C. pneumoniae can induce atherosclerosis when in the presence of elevated cholesterol levels. Seroepidemiological, histopathological, and animal model studies will continue as more of the pathophysiology of C. pneumoniae in atherosclerosis is uncovered.
While the pathogenesis of C. pneumoniae is still being studied, significant leads have been made in past years with the discovery of different factors that may contribute to the development of atherosclerotic plaques. The findings involving cLPS, chsp60, and their roles in LDL oxidation and foam cell formation are notable. The finding that cLPS was sufficient to induce DiI-LDL uptake into macrophages was important to the mechanism of foam cell formation. Chsp60 was found to induce monocyte LDL oxidation as well as activate human vascular cells, suggesting another mechanism for inflammation. cDNA microarray analysis was crucial in finding that endothelial cells infected with C. pneumoniae had up-regulated genes coding for inflammatory factors. While it is likely that the signaling pathways for cLPS and chsp60 overlap since similar cellular activities are invoked by both components, future experiments will dive further into understanding how these factors promote atherogenic events such as foam cell formation and LDL oxidation.
The idea that C. pneumoniae is involved in atherosclerosis is a difficult one to prove. Because the pathogenesis is extremely complicated, no single experiment will prove or disprove the idea. The accumulation of more evidence will build the knowledge on the bacteria's role in the disease. In the future, more well-defined studies on bacterial pathogenesis will add to this body of evidence. Identifying and testing immunological or therapeutic strategies that eradicate the bacteria in both respiratory and cardiovascular sites may be lead to the final answer.
References
Edited by Tara McIntyre, a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.
