Mississippi Dead Zone
Overview
By Kelly Poulos
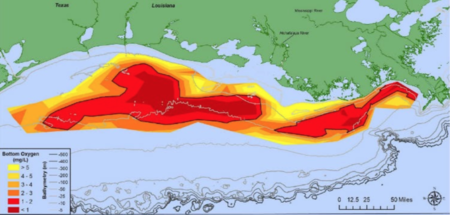
The Gulf of Mexico dead zone is an area of oxygen-depleted waters at the outflow of the Mississippi River. Dead zones are found worldwide, including in the Baltic Sea, Black Sea, and Chesapeake Bay, and are most common in the summer months.[2] In the case of the Gulf of Mexico, the dead zone is defined as being the portion of the water column with a dissolved oxygen concentration less than 2 milligrams per liter.[3] The record size of this hypoxic area is 8,776 square miles and it is caused largely by excess nutrient pollution into the Mississippi River.[4]

The excess of nutrients, largely nitrogen and phosphorus from runoff fertilizers and treated sewage discharge from urban areas, encourages the growth of algae and development of algal blooms.
In a natural system, the presence of these nutrients would not be harmful, however the increase in nitrogen and phosphorous due to anthropogenic factors contributes to a rapid growth of algae in a short period of time. When these algae die, they become a food source for bacteria, which consume dissolved oxygen as they decompose the algae, resulting in areas of the ocean with oxygen levels that are insufficient to support aquatic life, especially when there is strong stratification that prevents reoxygenation of the water column. As a result of this hypoxia, the microbial community in the water column shifts dramatically. This hypoxic zone can cover anywhere from 20%-50% of the Gulf of Mexico water column during the summer months and can persist through September.[7]
Detailed Environmental Description
The dead zone in the northern part of the Gulf of Mexico was first recorded in the early 1970s.[8] While the dead zone used to occur every couple of years, it is now a seasonal occurrence in the Gulf of Mexico. It is well researched as the dead zone is tied greatly to the fishing industry in the United States, however the microbial composition and metabolic potential of the microbes present in the dead zone is not well studied. Specifically, very little is known about the actual microbial ecology and physiology within the Gulf of Mexico dead zone. [9]
A connected issue to dead zones is that of agriculture and farming practices throughout the Midwestern United States. While the Gulf of Mexico dead zone has been present since the 1970’s, it has continued to grow in size each year. Until nutrient cycling and the transformation of these nutrients is addressed, the Gulf of Mexico dead zone will continue to be a seasonal norm.
Mississippi Dead Zone Microbial Ecology
Following the increase in nutrient load into the Gulf of Mexico from the Mississippi River, planktonic algae and phytoplankton populations boom as they feed off of the influx of nitrogen and phosphorus nutrients. Other common microorganisms that mark the first step of the formation of a dead zone, the growth of the algal bloom, include Cyanobacteria, Dinoflagellates, Coccolithophores, and Diatom algae.[9][10]
As these microbes die, their decomposition results in oxygen minimum zones, shifting the microbial community from one that is aerobic to one that is anaerobic. One study that looked at how the microbial composition shifts throughout the water column of an anoxic oxygen minimum zone found that microbes that perform anammox and sulfur oxidation become more abundant at greater depths of the dead zone [11]. As expected, the prevalence of aerobic microbes decreased in the core of the anoxic oxygen minimum zone.
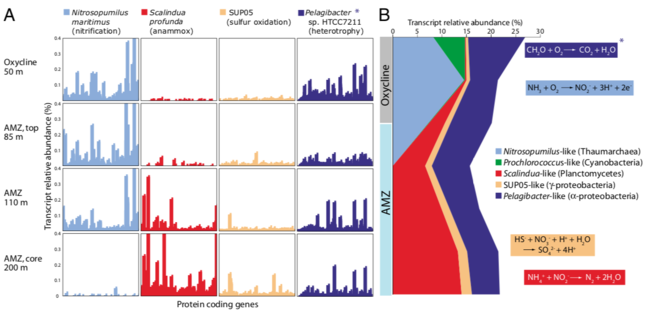
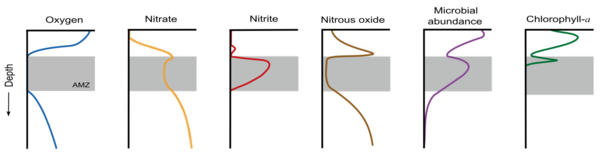
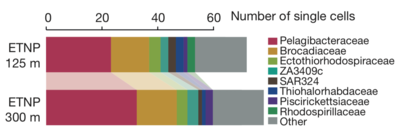
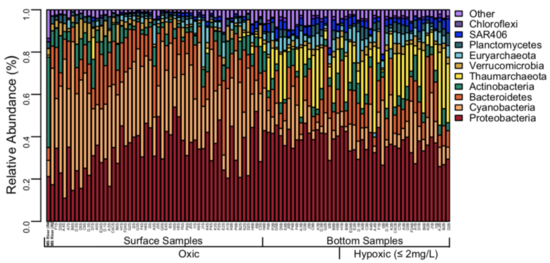
As the water column becomes anoxic in a dead zone, the biogeochemical profile shifts greatly. As available oxygen decreases, anaerobic metabolisms, such as nitrate and sulfate reduction and anaerobic ammonia oxidation become more prevalent.[12] In the absence of oxygen, there is still a substantial microbial community present in the water column along with increased nitrate and nitrate. The makeup of the water column shifts as a result of the formation of the dead zone. The image below illustrates this change in an anoxic oxygen minimum zone, which tend to have similar microbial profiles as dead zones given the shared absence of oxygen between the two.
Harmful Algal Blooms
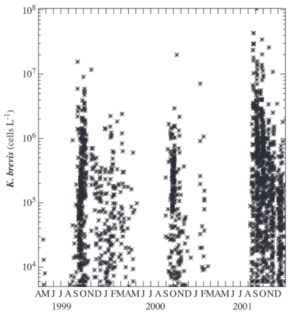
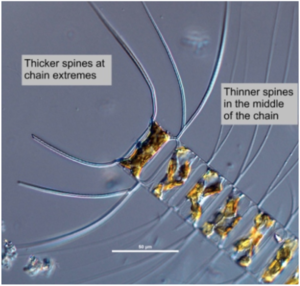
Harmful algal blooms (HAB) are common in the Gulf of Mexico and are the result of the overgrowth of toxic phytoplankton. One such example of a HAB is red tide where the overgrowth of microorganisms leads to the red discoloration of the surface waters. A well researched microorganism that is a common culprit of HABs is the dinoflagellate, Karenia brevi.[13]
Typically, cyanobacteria, microflagellates, and dinoflagellates are the organisms responsible for the formation of red tides and they kill marine species by paralyzing their respiratory systems. Algae species associated with these blooms produce potent toxins, which are liberated when eaten by other organisms. Other algae are capable of killing other organisms without toxins. For instance, the serrated spines of certain nontoxic algae, like that of certain Chaetoceros diatom species, can lodge in fish gills, leading to death.[15]
Other algae species found in HAB can form cysts that remain in sediment until environmental conditions are conducive to the occurrence of a bloom. These cysts are toxic to filter feeders like oysters. [15] The toxins created by these different microbes can bioaccumulate and impact humans.
Dead Zone Restoration
While fully restoring dead zones requires reducing nutrient delivery, the Gulf of Mexico dead zone typically lasts until late August or September when it is broken up by hurricanes or tropical storms. For the dead zone to be fully addressed, nutrient loads into the Mississippi River need to be limited. This, however, requires the collaboration of dozens of states and thousands of non-point source polluters. For dead zones to be eliminated, nutrient loads must be shrunk. Such plans have been undertaken in the Chesapeake Bay and in the Black Sea, which suffer from seasonal dead zones as well.[2] One method of addressing nutrient loads is to use a science-based approach where only the specific amount of fertilizer is applied to a given crop of land. Knowing the right type of fertilizer, amount of fertilizer, and time to apply the fertilizer can reduce nutrient runoff from farms into the Mississippi river and minimize the threat of the formation of a dead zone.
Key Microbial Players
SAR11
Alphaproteobacteria of the SAR11 clade of bacteria are abundant in oxygen minimum zones. They are aerobic heterotrophs that consume the dissolved organic carbon found in the ocean and transform it into carbon dioxide. A previous study found that SAR11 bacteria belonging to the family Pelagibacteraceae make up about twenty percent of all 16S rRNA genes and protein-coding metagenome sequences detected in an oxygen minimum zone.[7]
Thaumarchaeota
In the hypoxic zone, ammonia oxidizing archaea (AOA) are more common than ammonia oxidizing bacteria (AOB) because they can outcompete bacteria in low energy conditions. The AOA have a higher affinity for ammonia. Studies have found that there is often active ammonia oxidation and growth of AOA strains under low dissolved oxygen conditions.[10] One such ammonia oxidizing archaea that studies have found increase in abundance in low DO samples are Thaumarchaeota. These nitrifying microbes dominate the hypoxic zone of the Gulf of Mexico and contribute to N2O production in oxygen limiting environments. [8][10]
Conclusion
Understanding how the microbial community in the Gulf Mexico shifts as a result of increased nutrient loading and stratification, while is important to the tourism and fishing industries of the United States, plays a much larger role worldwide. It is predicted that dead zones will continue to expand in the face of climate change as expected increases in temperature will further enhance stratification in the water-column and thus prevent the reoxygenation of hypoxic zones. Apart from the anticipated temperature increases, climate models predict changes to rainfall patterns which, unless nutrient load is addressed, will contribute to higher amounts of nitrogen and phosphorus loading into the Mississippi River and therefore into the Gulf of Mexico. While these changes to the Gulf of Mexico can be expected in the face of climate change, the same can be said for dead zones worldwide. Being able to predict and potentially address shifts in the microbial communities of major water bodies worldwide could lead to further innovation and research to address growing dead zones.
References
- ↑ [Large 'dead zone' measured in Gulf of Mexico. (n.d.). Retrieved June 02, 2020, from https://www.noaa.gov/media-release/large-dead-zone-measured-in-gulf-of-mexico]
- ↑ 2.0 2.1 Mee, L. (2006). Reviving dead zones. Scientific American, 295(5), 78-85.
- ↑ [Ritzel, B. Gulf of Mexico’s Hypoxic Dead Zone.]
- ↑ [NOAA forecasts very large 'dead zone' for Gulf of Mexico. (n.d.). Retrieved June 02, 2020, from https://www.noaa.gov/media-release/noaa-forecasts-very-large-dead-zone-for-gulf-of-mexico]
- ↑ [Bruckner, M. (2019, October 15). The Gulf of Mexico Dead Zone. Retrieved June 02, 2020, from https://serc.carleton.edu/microbelife/topics/deadzone/index.html]
- ↑ [Gulf of Mexico Dead Zone. (n.d.). Retrieved June 02, 2020, from https://www.nature.org/en-us/about-us/where-we-work/priority-landscapes/gulf-of-mexico/stories-in-the-gulf-of-mexico/gulf-of-mexico-dead-zone/]
- ↑ 7.0 7.1 7.2 [Tsementzi, D., Wu, J., Deutsch, S., Nath, S., Rodriguez-R, L. M., Burns, A. S., ... & Stone, B. K. (2016). SAR11 bacteria linked to ocean anoxia and nitrogen loss. Nature, 536(7615), 179-183.]
- ↑ 8.0 8.1 Cartee, J. C. (2014). Significant changes in microbial community composition in the Gulf of Mexico" Dead Zone" over a diel cycle.
- ↑ 9.0 9.1 [Thrash, J. C., Seitz, K. W., Baker, B. J., Temperton, B., Gillies, L. E., Rabalais, N. N., ... & Mason, O. U. (2016). Decoding bacterioplankton metabolism in the northern Gulf of Mexico Dead Zone. bioRxiv, 095471.]
- ↑ 10.0 10.1 10.2 10.3 Campbell, L. G., Thrash, J. C., Rabalais, N. N., & Mason, O. U. (2019). Extent of the annual Gulf of Mexico hypoxic zone influences microbial community structure. PloS one, 14(4).
- ↑ 11.0 11.1 11.2 [Ulloa, O., Canfield, D. E., DeLong, E. F., Letelier, R. M., & Stewart, F. J. (2012). Microbial oceanography of anoxic oxygen minimum zones. Proceedings of the National Academy of Sciences, 109(40), 15996-16003. ]
- ↑ Thrash, J. C., Seitz, K. W., Baker, B. J., Temperton, B., Gillies, L. E., Rabalais, N. N., ... & Mason, O. U. (2017). Metabolic roles of uncultivated bacterioplankton lineages in the Northern Gulf of Mexico “dead zone”. MBio, 8(5), e01017-17.
- ↑ 13.0 13.1 [Tomlinson, M. C., Stumpf, R. P., Ransibrahmanakul, V., Truby, E. W., Kirkpatrick, G. J., Pederson, B. A., ... & Heil, C. A. (2004). Evaluation of the use of SeaWiFS imagery for detecting Karenia brevis harmful algal blooms in the eastern Gulf of Mexico. Remote Sensing of Environment, 91(3-4), 293-303. ]
- ↑ [The University of British Columbia. (n.d.). Retrieved June 02, 2020, from https://www.eoas.ubc.ca/research/phytoplankton/diatoms/centric/chaetoceros/c_decipiens_lorenzianus.html]
- ↑ 15.0 15.1 [Carlisle, E. (n.d.). The Gulf of Mexico Dead Zone and Red Tides. Retrieved June 02, 2020, from http://www.tulane.edu/~bfleury/envirobio/enviroweb/DeadZone.html
