Pyrocystis fusiformis
Classification
cellular organisms; Eukaryota; Alveolata; Dinophyceae; Pyrocystales; Pyrocystis [11]
Species
|
NCBI: Taxonomy |
Pyrocysits fusiformis
Description and Significance
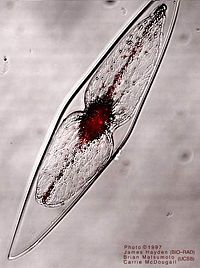
Pyrocysits fusiformis is a unicellular eukaryotic algae of the dinoflagellate phylum [1]. It is a protist species and being dinoflagellates have two flagella, one longitudinal and another transverse, for movement through water [10]. This species is a marine plankton with the ability to produce bioluminescence in response to water movement and high shear flow, usually around ships, breaking waves, or movement of predators[2].
Cells are fusiform shaped, elongated with tapered ends, and have an average length and width of 970 x 163 µm with the equivalent spherical diameter being 374 µm3 [1]. P. fusiformis has a cell wall but lacks a another sheathing of rigid polysaccharide plates that make up the cytoskeleton and are called thecae [1].
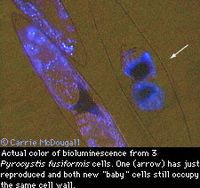
Bioluminescence of a blue color is produced instantaneously by this species when stimulated by movement, especially when cells are in high concentrations. Bioluminescence is depend on circadian rhythm or the cycle of light and dark during a 24 hour period; agitation during the day produces little bioluminescence and none is produced if cells are kept under constant lighted conditions [3]. Luminescence is most often produced by cells in the wake of ships, around swimming animals, or in breaking surface waves [2]. Bioluminescence is used by the organism as protection against predators by starling them with a flash of light or highlighting the movement of predators so that they are vulnerable to secondary predators. Both ways reduce the grazing pressure on P. fusiformis [3]. On average P. fusiformis can produce 23-62 flashes per second lasting 210 milliseconds with a maximum photon intensity of 690 x 109 photons per second (these values are for the first flash) [1]. Bioluminescence is stimulated by shear flow, velocity gradient, or low pH [5, 8].
The ability of P. fusiformis to instantaneously produce a bioluminescence when stimulated could prove a useful tool in flow visualization. This could be used in bioreactors to locate turbulent and dead zones[ 4]. This organism could also be used to study mechanical agitation caused by predators when feeding [1].
Genome Structure
The genome contains a high concentration of linear DNA that is tightly packed into permanently condensed chromosomes [12]. They lack nucleosomes as well as histones and the chromosomes form a liquid crystalline state in the nucleus [13]. This appears to be useful during replication. A well studied gene of this genome is luciferase made of 1242 amino acid residues [14]. It is highly conserved and has three tandem domains [14]. Also, it shares a common origin with other dinoflagellate luciferase genes [14]. The transcription mechanism as well as regulation for such a gene is unknown [13].
Cell Structure, Metabolism and Life Cycle
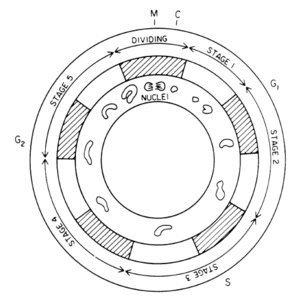
P. fusiformis undergoes several morphological changes during its cycle. It reproduces asexually generating one or two aplanospores or zoospores inside the cell wall [15]. These reproductive cells are not flagellated and increase rapidly to the size of the parent cell and become new cells [15]. What is of interest is that the chloroplasts change the cell shape, moving inward at night surrounding the nucleus and close to the cell wall during the day [7]. Scintillons are vacuoles that emit light and move opposite the chloroplasts movement.
P. fusiformis obtains energy from the sun through photosynthesis. In their environment, nitrogen is the limiting nutrient in the form of NO3- reduction to NH4+ and is taken up night and day in similar amounts [6]. During the dark period, the nitrogen gained accounts for higher carbon fixation rates during the day as well as a more stable C:N ratio [6].
Bioluminescence is emitted by a change in the fluidity of the plasma membrane, causing activation of GTP-binding proteins and a calcium flux [2]. An action potential is generated at the membrane surrounding the vacuole causing a proton flux which decreases cytoplasmic pH [2]. Luciferase is activated at the lower pH and allows a binding protein once associated with luciferin substrate to oxidize and produce light [2].
Ecology and Pathogenesis
Pyrocystis fusiformis is found in marine waters, often calm tropical or subtropical bays and can include oligotrophic regions. Populations peak at depths between 60 and 100 m where the light level is low [6]. P. fusiformis, along with other bioluminescent dinoflagellates, can use the ability to produce bioluminescence as an antipredation mechanism for protection. Bioluminescent is used to decrease grazing pressure and therefore increase survival by startling nearby predators with flashes of light [8]. Luminescence can be used to highlight the movement of organisms that graze on P. fusiformis, such as copepods, at night when they are invisible to predators. This makes the grazers vulnerable and visually detectable by its predators, thereby lowering the grazing pressure for dinoflagellates [3].
References
1. Latz, Michael I., Jennifer C. Nauen, and Jim Rohr. "Bioluminescence response of four species of dinoflagellates to fully developed pipe flow." Journal of Plankton Research 26 (2004): 1529-546.
2. Latz, Michael I., Michelle Bovard, Virginia Van Delinder, Enrico Segre, Jim Rohr, and Alex Groisman. "Bioluminescent response of individual dinoflagellate cells to hydrodynamic stress measured with millisecond resolution in a microfluidic device." The Journal of Experimental Biology 211 (2008): 2865-875.
3. Fleisher, K. J., and J. F. Case. "Cephalopod Predation Facilitated by Dinoflagellate Luminescence." The Biological Bulletin 189 (1995): 263-71.
4. McDougall, Carrie Ann. Bioluminescence and the actin cytoskeleton in the dinoflagellate Pyrocystis fusiformis: An examination of organelle transport and mechanotransduction. Diss. University of California, Santa Barbara, 2002. ProQuest. <http://proquest.umi.com.proxy2.cl.msu.edu/pqdweb?index=7&did=764917521&SrchMode=1&sid=1&Fmt=2&VInst=PROD&VType=PQD&RQT=309&VName=PQD&TS=1239677172&clientId=3552>.
5. Widder, Edith A., and James F. Case. "DISTRIBUTION OF SUBCELLULAR BIOLUMINESCENT SOURCES IN A DINOFLAGELLATE, PYROCYSTIS FUSIFORMIS." The Biological Bulletin 162 (1982): 423-48.
6. Bhovichitra, Mahn, and Elijah Swift. "Light and Dark Uptake of Nitrate and Ammonium by Large Oceanic Dinoflagellates: Pyrocystis noctiluca, Pyrocystis fusiformis, and Dissodinium lunula." Limnology and Oceanography 22 (1977): 73-78.
7. Sweeney, Beatrice M. "* Interaction of the Circadian Cycle with the Cell Cycle in Pyrocystis fusiformis." Plant Physiology 70 (1982): 272-76.
8. Maldonado, Eliza M., and Michael I. Latz. "Shear-Stress Dependence of Dinoflagellate Bioluminescence." The Biological Bulletin 212 (2007): 242-50.
9. Blaser, Stefan, Futoshi Kurisu, H. Satoh, and T. Mino. "Hydromechanical Stimulation of bioluminescent plankton." Luminescence 17 (2002): 370-80.
10. Madigan, Michael T., John M. Martinako, Paul V. Dunlap, and David P. Clark. Brock's Biology of Microorganisms. 10th ed. Benjamin Cummings.
11. National Center for Biotechnology Information. National Library of Medicine. <http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=295513>.
12. Seo, Kyung S., and Lawrence Fritz. "Karyology of a marine non-motile dinoflagellate, Pyrocystis lunula." Hydroniologia 563(2006):289-296.
13. Liu, Liyun and Woodland Hastings. "Novel and Rapidly Diverging Intergenic Sequences Between Tandem Repeats of the Luciferase Genes in Seven Dinoflagellate Species." J. Phycol 42(2005):96-103.
14. Liu,L., Wilson,T. and Hastings,J.W. "Molecular evolution of dinoflagellate luciferases, enzymes with three catalytic domains in a single polypeptide." Proc. Natl. Acad. Sci. U.S.A. 101 (47), 16555-16560 (2004)
15.Swift, Elijah and Edward Durbin. "Similarities in the Asexual Reproduction of the Oceanic Dinoflagellate, Pyrocystis Fusiformis, Pyrocystis Lunula, and Pyrocystis Noctiluca." J. Phycol 7(1971):89-96.
Author
Page authored by Fatima Foflonker and John Cowan, students of Prof. Jay Lennon at Michigan State University.
