Talimogene Laherparepvec: A groundbreaking viral therapy for late stage melanoma
By Alex Fazioli
Overview of Melanoma and effective treatment options
In 2015 an innovative treatment for late stage melanoma was approved due to excellent results in clinical testing with minimal side effects reported by patients. This new treatment for melanoma uses a genetically modified virus called Talimogene Laherparepvec (T-VEC). T-VEC has been genetically enhanced to target and destroy tumor cells while also increasing the bodies immune response to the melanoma. This one of a kind treatment is the first ever virus used to combat cancer in the United States and could possibly open the door for countless new viral treatments and combination therapies in the future. This page will focus on the clinical trials that led T-VEC to market and discuss the issues facing future viral therapies from being developed and approved.
Melanoma, one of the most dangerous forms of skin cancer, can occur when skin cells are exposed to UV radiation from the sun without protection [1]. When skin cells are frequently exposed to UV radiation, pigment-producing cells called melanocytes can accumulate mutations within their DNA, and sometimes these mutations can cause them to turn cancerous [1]. Cancerous cells divide unchecked by normal cell signaling and multiply into tumors on the skin [1]. Melanoma can occur anywhere on the human epidermis including the head, neck, hips, back, and even the eye [2]. The reason melanoma is considered to be dangerous is that it frequently becomes metastatic, which means that it spreads to areas around the human body different from where it originated, and when cancer spreads it becomes extremely difficult to treat [2]. One of the most common ways to see if someone has melanoma is to check their body for moles. The moles are often discolored, change in size and shape over time, and are asymmetrical [2]. Melanoma can be staged in 5 different categories, 0-IV, with the earlier stages being the easiest to treat while the later stages are the most difficult to treat especially if the cancer has spread throughout the human body.
In general, there are five different way of treating melanoma. The five most common techniques used are chemotherapy, surgery, immunotherapy, radiation therapy, and targeted therapy [2]. All of these treatments have positives and negatives associated with them, but this page will focus on one novel microbial based treatment for melanoma that falls within the immunotherapy category. One of the reasons to choose immunotherapy over other forms of treatment is that a variety of cancers can be treated because the immune system is being improved through the treatment [3]. Treatments such as radiation and chemotherapy are relatively ineffective against melanoma making immunotherapy one of the only viable options for patients with this illness [3]. Another benefit to immunotherapy is that its treatments have been shown to last longer in comparison to other therapies because the immune system is trained to recognize what cancer cells look like to eliminate them and inhibit tumor growth [3]. Immunotherapy is a more precise way to treat cancer than other common therapies such as chemo and radiation therapy which use radiation and toxic drugs that can be effective during treatment but cause extensive damage to normal human cells [3]. One of the most recent breakthroughs in immunotherapy was the development of the first Oncolytic virus called Talimogene Laherparepvec.
Development and mechanism of T-VEC
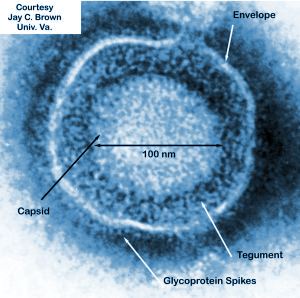
Herpes simplex virus (HSV) is a sexually transmitted disease that is the causative agent of herpes in humans (Figure 1). Some strains of HSV have been shown to naturally prefer replication within cancerous cells over healthy human cells (Figure 1)[4]. The ability of a virus to specifically replicate and destroy cancerous cells is of obvious importance to the medical and scientific worlds and countless researchers are trying to find strains of viruses that have shown this ability. The issue facing researchers is that most viruses do not solely target cancer cells, even if a preference for cancer specific replication has been shown. A solution to this issue is that cancer cells have a unique set of markers on their cell surface, different from normal human cells, that viruses can be genetically modified to target [4]. This was the idea that Biovex, the creators of T-VEC, had when when genetically engineering human herpes virus 1. T-VEC was modified to decrease its pathogenicity for healthy human cells while increasing its ability to attack and kill tumor cells [5].
The strain of HSV-1 used as a starting block for T-VEC was JS1, which exhibited a slight preference for cancer cell specific replication in comparison to other natural and engineered strains of herpes virus [6]. The JS1 strain of HSV-1 was genetically engineered by having its copies of the genes containing neurovirulence factors ICP34.5 and ICP47 deleted [6]. Neurovirulence factors give HSV-1the ability to cause disease in the human nervous system and the removal of those genes improves the safety of the virus. The removal of ICP34.5 specifically lowered the viruses neurotoxicity, allowing T-VEC to leave healthy human cells unharmed [6].
The removal of ICP47 is associated with an increase of the viruses ability to replicate with in cancer cells [6]. Increasing the ability of T-VEC to replicate within a cancer cell destroys them because the replication process takes up cellular energy, and once enough viral particles are made they will lyse the cell. The deletion of ICP47 is also associated with the upregulation of the gene US11 which also increases the viruses ability to replicate within cancerous cells [7].
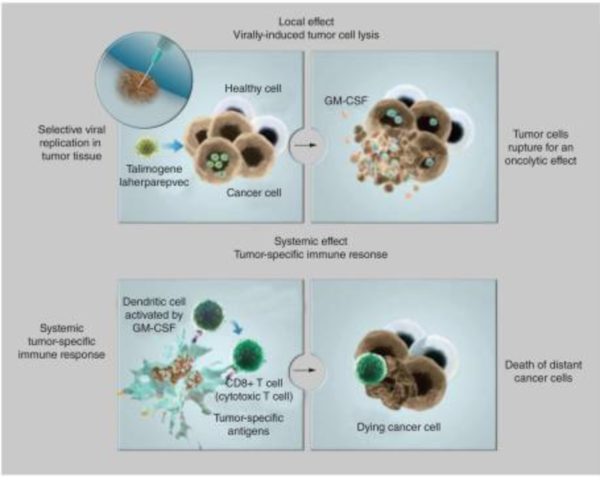
Finally, the gene for granulocyte macrophage colony-stimulating factor (GM-CSF) was inserted to replace the two deleted neurovirulence factors [7]. The insertion of GM-CSF allows T-VEC to increase immune response against cancer cells through the promotion of dendritic cells [7]. Dendritic cells are a part of the adaptive immune system and help signal immune responses by creating antigens that will signal the presence of cancerous cells to the human body. GM-CSF increases the ability of T-VEC to treat distal tumors not directly injected with the virus because the immune system is able to recognize cancer cells [7]. None of the alterations done by the BioVex group made T-VEC resistant to common antiviral drugs available such as acylclovir, which can be used to treat HSV-1 [5]. This gives doctors the ability to treat for a viral infection if something were to go wrong with T-VEC in a patient.
The mechanism of T-VEC is to selectively replicate within tumor cells and lyse them (Figure 2)[5]. Once the virus lyses a tumor cell many viral particles and tumor antigens will be released into the surrounding tumor and inducing a strong immune response against the tumor by signaling to the adaptive immune system which can fight cancerous cells (Figure 2)[6]. As the virus directly kills and lyses cancer cells, it spreads itself even further into the tumor by releasing replicated copies of its self, increasing its own effectiveness [6].
Phase I and II testing of T-VEC
Before T-VEC was able to move on to more important testing its developers needed to assess the safety of the therapeutic agent and see how effective it was at treating melanoma in live patients [8]. Overall, thirty patients took part in the first clinical trial of the drug and 13 of the patients were given a single dose of the drug while 17 patients got a multidose treatment of the drug [8]. The purpose of the multidose treatment was to see the impact of converting patients who had no trace of HSV-1 in their blood (seronegative) before receiving T-VEC. The multidose treatments started at a low concentration of T-VEC to avoid any serious side effects and the ensuing treatments went up in concentration allowing the seronegative patients to adapt to the virus [8]. In the single and multidose groups, the side effects seen most frequently were fever, nausea, vomiting, fatigue, and anorexia [8]. A common side effect seen in seronegative patients was a combination of inflammation and lumpy patches at the site of injection[8]. The process of starting the seronegative patients on a low dose of T-VEC and raising the concentration in subsequent treatments effectively allowed the patients to adapt to the new virus they had been exposed to, and the inflammation side effect went away in later treatments [8]. Other than inflammation and patchy skin the seronegative patients didn't experience any major side effects. None of the side effects seen in this study were considered to be intolerable by the patients thus allowing T-VEC to be considered safe enough for further testing.
The potency of the virus against tumors was also of interest to the researchers. The virus was found to be active against the tumors with evidence of replication, expression of GM-CSF, antigen-associated tumor necrosis, and tumor flattening [8]. All of these were the expected results of using T-VEC to treat melanoma. The impact of T-VEC against melanoma resulted in many of the tumors being shrunk in size and the progression of the disease in many patients was significantly slowed[8]. There was no significant difference in the ability of T-VEC to treat seronegative patients versus patients who already had been exposed to HSV-1 before treatment began [8]. The results of this study highlight T-VECs ability to specifically target cancerous cells when injected into a tumor. The therapy was able to shrink the size of tumors directly injected because of T-VECs ability to effectively infect cancerous cells and replicate rapidly inside of them (Figure 2).
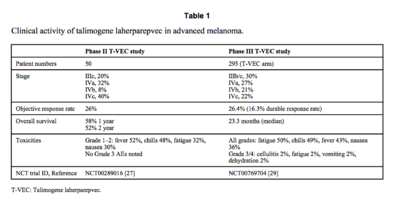
The goal of phase II testing was to see how effective T-VEC is in treating stage III melanoma that cannot not be removed by surgery and stage IV melanoma which has spread throughout the human body [2][9]. Both of these late stage melanomas are typically associated with low survival rates, with patients having a ~25% chance to survive for one year [8][9]. To see how effective T-VEC is at treating late stage melanoma, tumor size reduction was recorded and referred to as response rate and the survival rates of patients were recorded as well [9]. Overall, fifty patients took part in the study, 10 of which had stage III melanoma and the other 40 had various stage IV melanomas [9]. Seronegative patients were given a small initial dosage of T-VEC to adapt to the virus and then the concentration of the drug was increased in subsequent treatments [9]. Similar to the phase I study, no differences were seen in T-VECs ability to treat sernogative patients versus patients who had been exposed to HSV-1 before they began treatment. Tumors in eight of the patients were completely destroyed by the treatment of T-VEC and five of the patients had large portions of their tumors significantly reduced in size (Figure 3)[9]. T-VEC was not only effective in treating injected tumors but also at treating distant tumors that were not directly injected with the virus [9]. Reduction in the size of distant tumors highlights the ability of the T-VEC to stimulate an immune response against melanoma [9]. The duration of the treatment effect lasted on average for about half a year to just over two years for some patients [9]. This is important to note because part of the benefit of immunotherapy is a long lasting treatment. Patients with tumors that had a positive response to the T-VEC treatment were about twice as likely to survive a year than those who took part in other stage II clinical trials treating melanoma (Figure 3)[9]. The success of T-VEC in the phase II study combined with the proven safety profile of the drug from its phase I study evoked phase III testing of T-VEC.
Phase III testing of T-VEC and combination therapy
Various phase III studies of T-VEC were initiated after the promising results of phase I and II, one of which randomly compared T-VEC versus its immune stimulating component GM-CSF [11]. The goal of this study was to have its results, specifically the duration of treatment effect, to be reviewed by a blinded adjudication committee [11]. The point of a blind adjudication committee is to give unbiased expert opinions on the safety and effectiveness of new treatments and therapies. The duration of the treatment effect was defined by durable response rate, which was when a patient experienced a treatment response lasting longer than 6 months [11]. Other results analyzed in the phase III trial were tumor size reduction (objective response rate) and the overall survival rates of patients [11]. Two hundred and ninety five patients took part in the phase III study; roughly 30% had stage III melanoma and the other 70% had various stage IV melanomas (Figure 3)[11].
The overall survival rate of T-VEC patients was higher at 23.3 months versus the GM-CSF patients who survived on average 19 months (Figure 3)[11]. The results of the study show that the durable response rates were about 7 times higher for the T-VEC group than the GM-CSF group (Figure 3)[11]. The results of the study also showed that the T-VEC group had objective response rates about 4.5 times higher than the GM-CSF group. Tumor size reduction was seen in all the stages of melanoma being treated; however T-VEC was significantly more effective at treating the patients with earlier, less severe stages of melanoma such as stages III and IVa (Figure 3)[11]. The lowest survival rates and response rates were seen in the patients with the most advanced stages of melanoma [11]. The results of this report should not be taken lightly as T-VEC was the first ever oncolytic treatment to have positive results against late stages of melanoma.
The third stage of testing showed that T-VEC is most effective when it is directly injected into a tumor, maximizing its ability to replicate and lyse local tumor cells [11]. While T-VEC showed positive results against distant tumors not injected with T-VEC directly, those outcomes were not shown to be consistent in the third phase of testing, especially in patients with stages IVb/c (Figure 3)[11]. Where T-VEC lacks is in its ability to consistently stimulate an effective immune response to distant cancer cells [11]. This made researchers believe that T-VEC should only be used to treat late stage III and IVa melanoma (Figure 3). To increase the effect of T-VEC as an antitumor treatment, researchers believed they should pair it with other drugs that stimulate the immune system more effectively. One of the drugs researchers were interested in pairing T-VEC with was Ipilimumab, a drug that can activate the adaptive immune system by binding to proteins that have a negative effect on T-cells [11]. Combining T-VEC with Ipilimumab, both of which have aspects that improve the signaling of T-cells, could prove to be an effective anticancer treatment due to the ability of T-VEC to replicate and lyse tumor cells and Ipilimumab to draw out a more effective immune response [11][12].
While research is still continuing on the combination of immunotherapies, initial testing of T-VEC and Ipilimumab has already occurred and offered promising results. The combination of therapies was shown to be safe with no dangerous side effects [12]. Although initial testing of the combination therapy only had 19 patients, a very small sample size, the results of the study show that the combination of the two treatments may be more effective than each individual treatment [12]. Tumor size reduction in patients with stage III and IV melanomas were twice as high in the combination therapy than those seen in the T-VEC clinical testing (Figure 3)[11][12]. The durable response rates of the combination therapy were about 2.5 times higher than those seen in the T-VEC clinical testing [12]. The combination of T-VEC and Ipilimumab showed promising results with higher objective and durable response rates as well as higher overall survival rates [11][12]. The encouraging results of the phase I study justified a phase II randomized trial where a T-VEC + Ipilimumab treatment is compared to patients just treated with Ipilimumab [12]. The report on the phase II study of T-VEC combined with Ipilimumab is not out yet.
Issues Facing Oncolytic Viruses
Using oncolytic viruses creates special issues that normal drug manufacturers and creators do not have to face [13]. Oncolytic viruses are live viruses and can rapidly replicate within a patient once injected [13]. This is an issue because an effective dose for treatment may change depending on how the virus multiplies within the area of injection [13]. This impacts how much virus can be safely administered to patients for an effective treatment. This fact should also be taken into consideration when planning experiments using live viruses because injecting a small amount of virus can lead to an effective response because viral replication creates more viral particles that are released into the human body.
Another problem facing oncolytic viruses is safety protocols for the preparation, handling, and storage of the agent [13]. Since the viruses are live, they present a threat to anyone handling them because they can rapidly replicate within human cells [13]. If a virus infects and replicates within someone who handled the drug, there is always the possibility for adverse side effects even if genetic engineering has been done to increase the safety of the virus [13]. A single mutation could turn a therapeutic virus back into a pathogen that can cause major harm to the host.
Another issue for oncolytic viruses is their production on a large scale [13]. Viruses are different from other microbial organisms because they need live cells to replicate, which requires special methods to get the quantity of viruses needed for clinical trials and for producing the treatment for treat thousands of patients [13]. Extensive research has been done to create special procedures which ensure that any cells used to culture viruses are free from viral and microbial contaminants [14]. Issues within the clinical trial design and evaluation process are present too [13]. Evidence has been found suggesting a delayed immune response from some viral treatments that most clinical trials do not take into account [13]. Clinical trials must take into account the nature of their virus and set an appropriate time length to properly evaluate its effectiveness in treating disease. Another issue within the clinical testing is a lack of standardized assays showing the viruses distribution within the human body [13]. All trials should be required to provide evidence of the viruses presence within tumor cells, lack of presence within normal human cells, virus specific immune response, and the presence replicated viral particles being released from destroyed tumor cells [13]. Some clinical trials have done a good job in showing this evidence, but it has not yet become a standard practice for oncolytic virus testing [13].
In April of 2015 the FDA gave its approval of T-VEC, and officially licensed the drug for sale in October of 2015 [6][13]. T-VEC being approved by the FDA was significant not only because it is effective at treating late stage melanoma but because it opens up the door for countless other viral therapies and combination therapies [13]. One example of a new and promising combination therapie is T-VEC and pembrolizumab [13]. Outside of immunotherapy T-VEC can be combined with a wide range of other techniques used to treat cancer due to its tolerable safety profile. Some potential techniques to combine T-VEC with are chemotherapy, hormone therapy, surgery, and radiation therapy [13]. Although oncolytic viruses represent an untapped well of potential antiviral drugs, they face many problems coming to market in the US.
References
[1]Skin Cancer Foundation. Retrieved April 17, 2018, from https://www.skincancer.org/skin-cancer-information/melanoma
[2]Melanoma Treatment (PDQ®)-Patient Version. Retrieved April 18, 2018, from https://www.cancer.gov/types/skin/patient/melanoma-treatment-pdq
[3]Benefits of Cancer Immunotherapy. Retrieved April 24, 2018, from https://cancerresearch.org/immunotherapy/why-immunotherapy
[4]Russell, S. J., Peng, K. W., & Bell, J. C. (2012).Oncolytic virotherapy. Nature biotechnology, 30(7), 658.
[5] Dolgin, E. (2015). Oncolytic viruses get a boost with first FDA-approval recommendation. Nature Reviews Drug Discovery, 14(6), 369-371. doi:10.1038/nrd4643
[6] Pol, J., Kroemer, G., & Galluzzi, L. (2016). First oncolytic virus approved for melanoma immunotherapy. Oncoimmuneology, 5(1), e1115641 (3 pages)
[7]Liu, B. L., Robinson, M., Han, Z., Branston, R. H., English, C., Reay, P., & ... Coffin, R. S. (2003). ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Therapy, 10(4), 292.
[8] Hu, J. C., Coffin, R. S., Davis, C. J., Graham, N. J., Groves, N., Guest, P. J., ... & Medley, L. C. (2006). A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clinical cancer research, 12(22), 6737-6747.
[9] Senzer, N. N., Kaufman, H. L., Amatruda, T., Nemunaitis, M., Reid, T., Daniels, G., ... & Goldsweig, H. (2009). Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. Journal of Clinical Oncology, 27(34), 5763-5771.
[10] Korn, E. L., Liu, P. Y., Lee, S. J., Chapman, J. A. W., Niedzwiecki, D., Suman, V. J., ... & Parulekar, W. (2008). Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. Journal of Clinical Oncology, 26(4), 527-534.
[11] Johnson, D. B., Puzanov, I., & Kelley, M. C. (2015). Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy, 7(6), 611-619.
[12] Puzanov, I., Milhem, M. M., Minor, D., Hamid, O., Li, A., Chen, L., ... & Kaufman, H. L. (2016). Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. Journal of Clinical Oncology, 34(22), 2619-2626.
[13] Kaufman, H. L., Kohlhapp, F. J., & Zloza, A. (2015). Oncolytic viruses: a new class of immunotherapy drugs. Nature reviews Drug discovery , 14(9), 642.
[14] Chen, D. (2013). Safety assurance for biologics manufactured in mammalian cell cultures: a multitiered strategy. In Mammalian Cell Cultures for Biologics Manufacturing (pp. 167-183). Springer, Berlin, Heidelberg.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.
