User:Schuylerjones
WIKI IN PROGRESS
Ex. "Bordetella bronchiseptica"
Characteristics of the symbiont/pathogen

Bordetella bronchiseptica, stain RB50, is a small (0.4 by 8.0 um), gram negative, rod shaped beta-proteobacteria belonging to the Bordetellae family. "B. bronchiseptica" is nonsporeforming and pleomorphic, with or without flagella depending on the environment for which motility is needed. The average diameter of the flagella when present is 13.9 nm. "B. bronchiseptica" bacteria colonize within the respiratory tracts of mammals, commonly canines and felines, causing tracheobronchitis also known as "kennel cough". "B. bronchiseptica" is closely related to "Bordetella pertussis" and "Bordetella parapertussis", causing pertussis or whooping cough in humans [5]. "B. bronchiseptica" does not express the pertussis toxin, the virulence factor of "B. pertussis". However, "B. bronchiseptica" has the genes to express the toxin which shows that "B. bronchiseptica" is closely related to "B. pertussis" [7].
"B. bronchiseptica" consists of 5,339,179 base pairs with 5,011 coding sequences. Its average gene size is 982 bp and it has 18 pseudogenes. The gene responsible for virulence is BvgAS [9].
Characteristics of the host
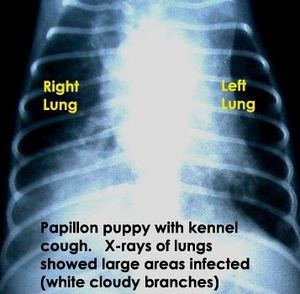
"Bordetella bronchiseptica" can infect a wide range of hosts causing different diseases and is most commonly found in canines, pigs, and laboratory animals [5]. "B. bronchiseptica" infects its host by attaching to hamster lung fibroblasts when inhaled [7]. The bacterium is capable of living outside of a host most likely in areas where animals are kept in close confinement. "B. brochiseptica" causes swine atrophic rhinitis and pneumonia in pigs, deforming and stunting the growth of the turbinates in the snout. "B. brochiseptica" is the primary pathogen of swine. "B. bronchiseptica" causes acute tracheobronchitis, otherwise known as kennel cough, in cats and dogs. It is unknown whether "B. bronchiseptica" is passed from farm to companion animals [5] and humans are rarely infected [7]. "B. parapertussis" and "B. pertussis" have less autotransporter genes and a higher number of autotransporter pseudogenes than "B. bronchiseptica". This difference in autotransporter proteins could possibly contribute to the bacteria's host specificity and the different diseases they cause [6].
Host-Symbiont Interaction
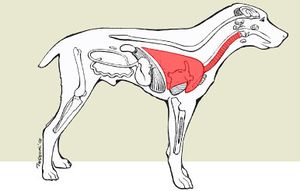
The interaction between "B. bronchiseptica" and its host is pathogenic. Infection of "B. bronchiseptica" causes kennel cough in canines and is of high morbidity and low mortality [5]. The bacterium is characterized as a facultative parasite in which it is capable of surviving for an extensive amount of time outside of a host. The host acquires "B. bronchiseptica" when exposed to an infected surface, specifically where an already infected host has been. Usually canines come in contact with the bacterium when boarded in a close confined area such as a kennel. The host inhales "B. bronchiseptica" and colonizes on the muccous membranes lining the respiratory tract and produces factors that counteract the host's defense mechanisms. "B. bronchiseptica" infects healthy ciliated epithelial cells whereas most respiratory pathogens cannot. A host transmits the symbiont by coughing and therefore infected a surface. As the host's defenses become weak, they are vulnerable to other infections. [10].
Molecular Insights into the Symbiosis
"B. bronchiseptica" expresses many protein factors, such as filamentous haemagglutinin (FHA), pertactin and fimbriae. Specifically, FHA binds directly to the ciliary membrane host-receptor glycospingolipids, allowing "B. bronchiseptica" to attach to the host's cells. "B. bronchiseptica" also produces many toxins such as bifunctional adenylate cyclase and dermonecrotic toxins. All of the virulence factors of "B. bronchiseptica" allow it to weaken the host's immune defenses, leading to infection. BvgA and BvgS, two-component regulatory systems, regulate these virulence factors. BvgS isa histidine kinase sensor on the inner membrane. BvgS auto-phosphorylates before it phosphorylates the BvgA protein. Transciption is then followed by this process and specific virulent genes are expressed by binding to their promoter sites. [7]
Ecological and Evolutionary Aspects
"B. bronchiseptica" was isolated in 1910 by a man named Ferry. "Bordetella" spp. are aerobic in which they require oxygen to survive. Optimal growth for all "Bordetella" spp. is at 35 to 37 degrees Celsius. Intracellular survival of "B. bronchiseptica" is extremely controversial, however it is known that the bacterium can survive for long periods of time without a host. While living inside the host the bacterium is capable of living for about 1 to 3 weeks. The bacterium thrives in areas of close confinement such as breeder and boarding kennels. Due to the fact that the bacterium transmits through the cough of the infected host, it is highly contagious. Hosts that have previously been infected are more immune to the bacterium. In contrary, infection rates may be less frequent outside of close confined areas [8].
Isolates of "B. bronchiseptica" from the Netherlands and the United States have shown several dissimilar electrophoric types. Certain electrophoric types have been found in Europe but not in the United States, where some electrophoric types may be found only in a specific geographic region while others are more common. [11].
Recent Discoveries
One recent study was done to examine the significance of FHA and PRN in "B. bronchiseptica". Deletions of the fhaB gene or the prn gene of KM22 were observed in swine isolates and KM22 was found to be an appropriate gene to be present a reproducible swine model among "B. bronchiseptica" infections. Protein expression of strain TN27 and TN28 was done through Western plot analysis. The prn gene was present in KM22 and TN27 whereas BvgA was present in all three strains. ~240-kDa polypeptide was also found in all three strains. Overall, it was concluded that the FHA and PRN is absent in the TN27 and TN 28 strain. Moreover, PRN does not contribute to the symptoms and pathology, whereas FHA contributes to host specificity [11].
Another recent study discovered that the outermembrane protein BcfA encourages colony growth on the trachea. Data also indicated that BcfA -specific antibodies were present in infected hosts. Furthermore, the use of BcfA to immunize causes immunity against "B. bronchiseptica" infections [12].
References
[[5] Binns, S. H., Corkill, J. E., Dawson, S., Gaskell, R. M., Hart, C. A., Kariuki, S., Osborn, A. M., Saunders, J. R., and Speakman, A. J. 1997. Characterization of antibiotic resistance plasmids from "Bordetella bronchiseptica". "Journal of Antimicrobial Chemotherapy". 40: 811-816.]
[[6] Appel J. G. M., Bemis, A. D., and Greisen, A. H. 1977. Pathogenesis of canine bordetellosis. "The Journal of Infectious Diseases". 135: 753-762.]
[[7] Vanderzee, A, Mooi, F., Emben, J. V., and Musser, J. Molecular evolution and host adaptation of "Bordetella" spp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. 1997. "Journal of Bacteriology". 179: 6609–6617.]
[[8] Roberts, M., and Stevenson, A. 2003. Use of "Bordetella bronchiseptica" and "Bordetella pertussis" as live vaccines and vectors for heterologous antigens. "FEMS Immunology and Medical Microbiology". 37: 121-128.]
[[9] Arico, B. and Rappuoli, R. "Bordetella parapertussis" and "Bordetella bronchiseptica" contain transcriptionally silent pertussis toxin genes. 1987. "Journal of Bacteriology". 169: 2847-2853.]
[[10] Yuk, M. H., Harvill, E. T., and Miller, J. F. 1998. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. "Molecular Microbiology". 28: 945-959.]
[[11] Nicholson, T. L., Brockmeier, S. L., and Loving, C. L. 2009. Contribution of "Bordetella bronchiseptica" filamentous hemagglutinin and pertactin to respiratory disease in swine. "Infection and Immunity". 77: 2136–2146.]
[[12] Sukumar, N., Love, C. F., Conover, M. S., Kock, N. D., Dubey, P., and Deora, R. 2009. Active and passive immunizations with "Bordetella" colonization factor A protect mice against respiratory challenge with "Bordetella bronchiseptica". "Infection and Immunity". 77: 885-895.]
Edited by [Schuyler Jones], students of Grace Lim-Fong
