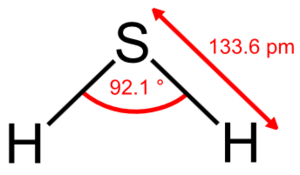Vinification, flavor, and aroma: Difference between revisions
No edit summary |
No edit summary |
||
| (10 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
[[Image:Rahovec_Grapes_and_Wine.JPG|thumb|512px|right|Photo of Rahovec Grapes, a common fruit used for vinification.]] | [[Image:Rahovec_Grapes_and_Wine.JPG|thumb|512px|right|Photo of Rahovec Grapes, a common fruit used for vinification.]] | ||
Wine making or [https://microbewiki.kenyon.edu/index.php/Vinification vinification] is a process through which juice, typically grape, is fermented into an alcoholic beverage. Vinification is a complex multistep process, with many uncontrollable variables to be accounted for. Vinification requires the careful management of the microflora present, nutrient availability for the microflora, pH, temperature, and storage conditions, in order to make a palatable vintage. [http://en.wikipedia.org/wiki/Viticulture Viticulture] refers to the manage the soil, vines, fruit health and harvest, while [http://en.wikipedia.org/wiki/Oenology enology] refers to all other aspects of the process that turn the juice to wine. Viticulture and enology research support a demand within the [http://en.wikipedia.org/wiki/Wine wine] industry to develop scientifically supported practices for managing problems in the field. [http://en.wikipedia.org/wiki/Varietal Varietal] refers to the grape type, such as a [http://en.wikipedia.org/wiki/Chardonnay Chardonnay] or [http://en.wikipedia.org/wiki/Durif Petite Sirah], and each varietal | Wine making or [https://microbewiki.kenyon.edu/index.php/Vinification vinification] is a process through which juice, typically grape, is fermented into an alcoholic beverage. Vinification is a complex multistep process, with many uncontrollable variables to be accounted for [1,2,3,4]. Vinification requires the careful management of the microflora present, nutrient availability for the microflora, pH, temperature, and storage conditions, in order to make a palatable vintage. [http://en.wikipedia.org/wiki/Viticulture Viticulture] refers to the manage the soil, vines, fruit health and harvest, while [http://en.wikipedia.org/wiki/Oenology enology] refers to all other aspects of the process that turn the juice to wine. Viticulture and enology research support a demand within the [http://en.wikipedia.org/wiki/Wine wine] industry to develop scientifically supported practices for managing problems in the field. [http://en.wikipedia.org/wiki/Varietal Varietal] refers to the grape type, such as a [http://en.wikipedia.org/wiki/Chardonnay Chardonnay] or [http://en.wikipedia.org/wiki/Durif Petite Sirah], and each varietal is endemic to different climates. Ultimately though, due to the value of a [http://en.wikipedia.org/wiki/Wine_fault palatable wine], the science of vinification is most used to improve the [http://en.wikipedia.org/wiki/Flavor flavor] and [http://en.wikipedia.org/wiki/Aroma_compound aroma] of the final product. | ||
The perception of a wine’s flavor and aroma comes from a multitude of interactions between about a 1,000 chemical compounds within the wine and our own sensory receptors. These chemical compounds function in many different ways, and can act synergistically or antagonistically with each other. Flavor and aroma compounds are derived from the grapes themselves, the wood fermentation occurs in, and the [http://en.wikipedia.org/wiki/Metabolite metabolites] of microbes used in the fermentation process. Making a tasty wine is largely related to the ratios of volatile compounds found in the wine; wines | The perception of a wine’s flavor and aroma comes from a multitude of interactions between about a 1,000 chemical compounds within the wine and our own sensory receptors [1,3,8]. These chemical compounds function in many different ways, and can act synergistically or antagonistically with each other [1,3]. Flavor and aroma compounds are derived from the grapes themselves, the wood fermentation occurs in, and the [http://en.wikipedia.org/wiki/Metabolite metabolites] of microbes used in the fermentation process [1,2,3]. Making a tasty wine is largely related to the ratios of [http://en.wikipedia.org/wiki/Volatile_organic_compound volatile compounds] found in the wine; wines without enough of a certain compound will lack flavor and fullness, while wines with too much of a compound can taste spoiled [1,2,3,4,5,8]. These compounds result from both the alcoholic fermentation process and the malolactic fermentation, which are each driven by their own microbes [2]. | ||
Biological and chemical principles guide the science behind the entire vinification process. Once fruit is harvested, it is the winemakers duty to ferment the juice into a palatable wine through complex interactions of yeast and bacteria. Fermentation in wine is achieved primarily by the yeast [http://en.wikipedia.org/wiki/Yeast Saccharomyces cerevisiae] and secondarily through fermentation by malolactic bacteria. Grapes in the field, and in vats after harvest contain natural amounts of residual nutrients, bacteria, and yeast. The [http://en.wikipedia.org/wiki/Must grape must] is a combination of juice, seeds, skins, and stems, along with a host of microbes. Upon crushing the grapes, the juice is naturally inoculated with the yeasts and bacteria from the must. Common industry practice dictates inoculating juice with yeast from a cultured stock bred for vinification to dominate the fermentation process in a favorable way. To determine the length of the fermentation, along with what nutrients to add, enologist assess the biochemical state of the wine throughout fermentation. Concern is paid to particular bacteria, and yeast strains associated with [http://en.wikipedia.org/wiki/Food_spoilage spoilage]. Spoilage refers to the presence of compounds associated with off putting aroma and flavor and is explained in more depth in section 2. More important than identifying problematic microbes, is thorough management of the microbial environment of the wine. The proper microbial environment during fermentation ensures that the intended organisms thrive and outcompete spoilage microbes. This includes measuring pH, sugar (or [http://en.wikipedia.org/wiki/Brix brix]), [http://en.wikipedia.org/wiki/Yeast_assimilable_nitrogen Yeast assimilable nitrogen] (YAN), and [http://en.wikipedia.org/wiki/Free_amino_nitrogen Free Amino Nitrogen] (FAN) discussed in section three, which all help manage spoilage. | Biological and chemical principles guide the science behind the entire vinification process. Once fruit is harvested, it is the winemakers duty to ferment the juice into a palatable wine through complex interactions of yeast and bacteria [1,2,8]. Fermentation in wine is achieved primarily by the yeast [http://en.wikipedia.org/wiki/Yeast Saccharomyces cerevisiae] and secondarily through fermentation by malolactic bacteria [1,2,5-7]. Grapes in the field, and in vats after harvest contain natural amounts of residual nutrients, bacteria, and yeast [1,2]. The [http://en.wikipedia.org/wiki/Must grape must] is a combination of juice, seeds, skins, and stems, along with a host of microbes. Upon crushing the grapes, the juice is naturally inoculated with the yeasts and bacteria from the must. Common industry practice dictates inoculating juice with yeast from a cultured stock bred for vinification to dominate the fermentation process in a favorable way [2]. To determine the length of the fermentation, along with what nutrients to add, enologist assess the biochemical state of the wine throughout fermentation [1,2,3,8] . Concern is paid to particular bacteria, and yeast strains associated with [http://en.wikipedia.org/wiki/Food_spoilage spoilage]. Spoilage refers to the presence of compounds associated with off putting aroma and flavor and is explained in more depth in section 2. More important than identifying problematic microbes, is thorough management of the microbial environment of the wine. The proper microbial environment during fermentation ensures that the intended organisms thrive and outcompete spoilage microbes [2]. This includes measuring pH, sugar (or [http://en.wikipedia.org/wiki/Brix brix]), [http://en.wikipedia.org/wiki/Yeast_assimilable_nitrogen Yeast assimilable nitrogen] (YAN), and [http://en.wikipedia.org/wiki/Free_amino_nitrogen Free Amino Nitrogen] (FAN) discussed in section three, which all help manage spoilage [1,2,3,8]. | ||
The page is aimed at a general overview of vinification practices and research into these practices that deal with flavor and aroma. That includes a brief overview of the microbial activity in vinification (alcohol and malolactic) to understand some of the general organisms involved in the process. One the basic fermentation process is outlined in a biological context, it is important to what compounds in wine are important to flavor and aroma, and how the compounds result from microbial metabolism. This includes covering flavor modulation by yeast, and the formation of hydrogen sulfide (spoilage) compounds. Last, common practical applications for altering the flavor compounds or microbial processes in wine are discussed. | The page is aimed at a general overview of vinification practices and research into these practices that deal with flavor and aroma. That includes a brief overview of the microbial activity in vinification (alcohol and malolactic) to understand some of the general organisms involved in the process. One the basic fermentation process is outlined in a biological context, it is important to what compounds in wine are important to flavor and aroma, and how the compounds result from microbial metabolism. This includes covering flavor modulation by yeast, and the formation of hydrogen sulfide (spoilage) compounds. Last, common practical applications for altering the flavor compounds or microbial processes in wine are discussed. | ||
| Line 15: | Line 15: | ||
===Alcoholic Fermentation=== | ===Alcoholic Fermentation=== | ||
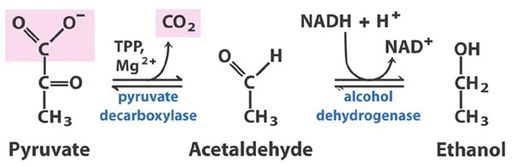
[[Image:Pyruvate_decarb_1.jpg|thumb|512px|right|Illustration showing how pyruvate, broken down from glucose, is metabolized to form carbon dioxide and ethanol.]] | [[Image:Pyruvate_decarb_1.jpg|thumb|512px|right|Illustration showing how pyruvate, broken down from glucose, is metabolized to form carbon dioxide and ethanol.]] | ||
[http://en.wikipedia.org/wiki/Ethanol_fermentation Alcohol fermentation] is the process through which sugars are converted to ethanol and carbon dioxide. | [http://en.wikipedia.org/wiki/Ethanol_fermentation Alcohol fermentation] is the metabolic process through which sugars are converted to ethanol and carbon dioxide (Micro text). Ripe grapes contain high levels of the simple sugars fructose and glucose (~15%) which are quickly metabolized into pyruvate by yeast [2]. Pyruvate is then used in the biosynthesis of essential constituents like amino acids, and fatty acids, with by products of carbon dioxide, diacetyl, ethanol, esters, and other long chain alcohols [2]. See the figures for additional guidance in the process. Figure two has a description of the fundamental chemical changes in fermentation, figure three shows a chart of the major chemicals produced in wine fermentation. It is these chemicals along with variations in other flavor compounds formed during vinification that make make up the sensory quality of a wine [3,8]. Yeast, mainly Saccharomyces cerevisiae can ferment in aerobic and anaerobic conditions, and is the main contributor to alcohol formation because of its tolerance to high ethanol concentrations (ethanol tolerance in yeast 1986). Other yeasts naturally found in the grape must include Kloeckera and Hanseniaspora [1,2]. S. cerevisiae is shown to modify lipids structures to endure the stress of an ethanol environment [9]. Typically the inoculated yeast populations dominate the later stages of fermentation, a period lasting about 6-20 days depending on initial sugar concentration, or until ethanol reaches about 15% and yeast growth stops [1,2]. | ||
===Malolactic Fermentation=== | ===Malolactic Fermentation=== | ||
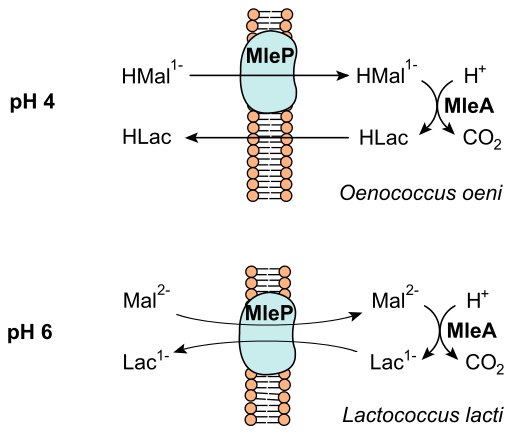
[[Image:Malolactic_fermentation.png|thumb|512px|right|Illustration showing chemical process of malolactic fermentation in two different lactic acid bacteria (O. oeni and L. lacti).]] | [[Image:Malolactic_fermentation.png|thumb|512px|right|Illustration showing chemical process of malolactic fermentation in two different lactic acid bacteria (O. oeni and L. lacti).]] | ||
The secondary phase of fermentation is known as [http://en.wikipedia.org/wiki/Malolactic_fermentation malolactic fermentation]. Malolactic fermentation is the decarboxylation of L-malic acid to L-lactic acid through enzymatic activity. Several lactic acid bacteria can be responsible for malolactic fermentation, with varying effects on the wines flavor and aroma. | The secondary phase of wine fermentation is known as [http://en.wikipedia.org/wiki/Malolactic_fermentation malolactic fermentation]. It occurs in all red wines, and some whites. After fermentation, the wine is drained from the sediment and yeast, and put into various containers. This is the point in the fermentation process in which wood might be introduced in the form of a wooden barrel, while other winemakers select stainless steel depending on the desired taste [1,2,5-7]. In some cases, wine is fermented a second time by malolactic fermentation. Malolactic fermentation is the decarboxylation of L-malic acid to L-lactic acid through enzymatic activity [2]. This chemical process decreases the acidity from the malic acid in grapes by converting malic acid to L-malate which is fermented by Oenococcus oeni [1,2,5]. See figure four for a illustration of this chemical process. Several lactic acid bacteria can be responsible for malolactic fermentation, with varying effects on the wines flavor and aroma. Lactic acid bacteria in grape must come from the genera Lactobacillus, Pediococcus, Leuconostoc, and Oenococcus. Citric and malic acid can also be fermented by the lactic acid bacteria known as Oenococcus oeni [5,6,7]. The bacteria can be bought to be added manually, but they also occur naturally. The process of citric acid fermentation produces diacetyl, a compound associated with nutty and buttery aromas in wine [2,5]. Sulfite is commonly added to the wine after malolactic fermentation to stop microbial activity, which may prohibit the wine from completing the degradation of citric acid [2,5]. The bacteria can contribute to aroma and flavor of wine by the process of malolactic fermentation where grape derived secondary metabolites can be hydrolyze glycosylated aroma precursors [9]. | ||
Citric and malic acid can also be fermented by the lactic acid bacteria known as | |||
Certain spoilage bacteria give wine off flavors and poor aroma [1,2,8]. These can be bacteria or molds that grow within in wine [http://en.wikipedia.org/wiki/Cork_taint corks] [10]. Growth of the bacteria [http://en.wikipedia.org/wiki/Pediococcus Pediococcus spp] can have negative impacts on wine flavor and aroma [1,2,5-7]. An example is the formation of diacetyl and acetoin. Growth of the [http://en.wikipedia.org/wiki/Lactobacillus Lactobacillus spp] can also have negative impacts on flavor and aroma. Uncontrolled growth of the species is associated with volatile acidity and the formation of compounds like diacetyl, acetoin, and Heresztyn [11]. Sulfur Dioxide is commonly used to control the growth of the aforementioned undesirable bacteria [1,2]. Sulfur dioxide and the pH of win inhibit Lactobacillus spp growth [2,5,6,11]. Though some Pediococcus and Lactobacillus contribute to favorable flavor characteristics, excessive growth of the bacteria will cause spoiling [11]. | |||
<br> <br> | <br> <br> | ||
| Line 36: | Line 29: | ||
==Compounds That Effect Flavor and Aroma== | ==Compounds That Effect Flavor and Aroma== | ||
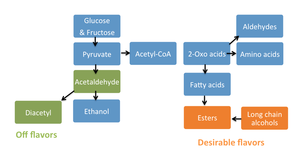
Wine aroma and flavor is caused by the presence of various compounds intrinsic to the climate and fermentation process. | Wine aroma and flavor is caused by the presence of various compounds intrinsic to the climate and fermentation process. The low pH and high sugar content of grapes exerts a strong selective regime on microorganism within the wine, so that only certain yeast and bacteria species are able to thrive [2,8,12]. The selection regime for a fermenting must only strengthens in anaerobic conditions. The roles individual bacteria and yeast species play in fermentation are directly tied to the taste and smell of the wine as a final product [1,3,4]. The following section outlines in depth the flavor and aroma compounds that give a wine its complexion, and describes how briefly how they are synthesized. Many flavor and aroma compounds contribute beneficially to a wine at some level, but at to high concentration they produce an off flavor. Some of the main compounds and how they relate to fermentation are described in figure 4. | ||
[[Image:figure 4.png|thumb|300px|right|Modified fermentation diagram from Microbiology: An Evolving Science to show the metabolic pathways that yield flavor and aroma compounds in wine.]] | |||
===Flavor modulation by yeast=== | ===Flavor modulation by yeast=== | ||
The following 16 yeast species are common in wine making process: Brettanomyces, Dekkera, Candida, Cryptococcus, Debaryomyces, Hanseniaspora and its asexual counterpart Kloeckera, Kluyveromyces, Metschnikowia, Pichia, Rhodotorula, Saccharomyces, | The following 16 yeast species are common in wine making process: Brettanomyces, Dekkera, Candida, Cryptococcus, Debaryomyces, Hanseniaspora and its asexual counterpart Kloeckera, Kluyveromyces, Metschnikowia, Pichia, Rhodotorula, Saccharomyces, Saccharomyces, Schizosaccharomyces, Torulaspora and Zygosaccharomyces [1]. Throughout the fermentation process different yeast thrive or dwindle according to nutrient availability and environmental conditions. Kloeckera, Hanseniaspora and Candida are shown to thrive in early stages. Several species of Metschnikowia and Pichia follow as concentrations rise. During the latter stages of fermentation in higher alcohol concentrations, Saccharomyces cerevisiae thrives [1,3]. | ||
====Non-volatile acids==== | |||
Non volatile acids influences the growth of microbes, and the overall chemistry of the wine. It is responsible for characteristics like ‘freshness’ among certain varietals. The acidity of grapes is dependent on the temperature of the region [1,2,4]. Some acid species found in wine during the fermentation process include succinic acid, keto acids (pyruvic and ketoglutaric acid), lactic acid, and malic acid. Succinic acid, associated with yeast fermentation, is known to have a bitter and overly salty taste [1,2]. | |||
====Volatile organic acids==== | |||
Volatile organic acids are also major in wine chemistry. Among the volatile acid species are acetic acid (90%) and the rest comprised of propionic and hexanoic acid. They result from fatty acid metabolism in yeast and bacteria. Acetic acid can impart a vinegar taste at high concentrations. Acetate is produced by yeast, but most acetic acid in wine comes from the metabolism of ethanol by aerobic acetic acid bacteria [1,2]. | |||
Alcohols – ethanol is the main alcohol of interest in wine. High sugar levels in fruit convert to wines with more ethanol. Enough ethanol enhances the sensory perception of a wine, but too much produces a burn that can mask the wine [1,2,3,4]. | |||
====Glycerol==== | |||
Glycerol is another product of the alcoholic fermentation process. Glycerol is a polyol that tasts slightly sweet with an oily mouth feel. Glycerol is found in dry and semi-sweet wines at concentrations of 5-14 g/L [1]. In red wines glycerol concentrations are higher than in whites. Glycerol metabolism is very important to the anaerobic fermentation of sugars because it plays key roles in cell growth and division [1]. | |||
Higher alcohols or fusel alcohols are secondary yeast metabolites. Higher alcohols can have beneficial or detrimental effects on wine flavor and aroma. Too much of a higher alcohol gives wine a strong pungent smell, but the right amount contributes to a wines fruit notes [1,2,3]. Higher alcohol formation can be variable among different yeast strains. Yeast products include acetaldehyde, pyruvic acid, and vinyl phenol [1,2,3]. | |||
Another compound important to taste is diacetyl which in smaller concentrations gives wine a buttery or toasty flavor, but at high concentrations it is off putting. Most diacetyl in wine comes from the metabolic activities of lactic acid bacteria [1,2]. Other compound varieties include volatile phenols, esters, sulfur compounds, sulfides, thiols and monoterpenoids [1,2,4]. | |||
===Hydrogen Sulfide=== | ===Hydrogen Sulfide=== | ||
| Line 63: | Line 54: | ||
[[Image:Hydrogen-sulfide-2D-dimensions.png|thumb|300px|right|Diagram of a hydrogen sulfide molecule with bond angles included.]] | [[Image:Hydrogen-sulfide-2D-dimensions.png|thumb|300px|right|Diagram of a hydrogen sulfide molecule with bond angles included.]] | ||
Yeast has role in production of volatile sulfur compounds through the sulfate reduction sequence pathway. The importance of sulfur compounds in wine has a tremendous effect on the sensory quality of the wine. Hydrogen sulfide can make wines smell rotten while other sulfur compounds like the flavor active thiol 3-mercaptohexanol, can impart fruity notes beneficial to the sensory quality of a wine. The wine yeast Saccharomyces cerevisiae is responsible for forming many of these volatile sulfur compounds that contribute to a wines sensory quality. | Yeast has role in production of volatile sulfur compounds through the sulfate reduction sequence pathway [1,2,13]. The importance of sulfur compounds in wine has a tremendous effect on the sensory quality of the wine. Hydrogen sulfide can make wines smell rotten while other sulfur compounds like the flavor active thiol 3-mercaptohexanol, can impart fruity notes beneficial to the sensory quality of a wine [13]. The wine yeast Saccharomyces cerevisiae is responsible for forming many of these volatile sulfur compounds that contribute to a wines sensory quality [1,13]. | ||
Hydrogen sulfides found in wine are formed through the metabolic pathways of yeast from inorganic sulfur compounds, sulfate and sulfite, and from organic sulfur compounds cysteine and glutathionine. Hydrogen sulfide is released from the cell as result of the sulfate reduction sequence pathway. Hydrogen sulfide is derived from the HS- ion, which serves as an intermediate stage in the reduction of sulfite and sulfate to synthesize organic sulfur compounds. The HS- ion is sequestered in the presence of a nitrogen supply to form methionine and cysteine. Thus in low nitrogen environments, sulfide gathers within the cell and be released as hydrogen sulfide. | Hydrogen sulfides found in wine are formed through the metabolic pathways of yeast from inorganic sulfur compounds, sulfate and sulfite, and from organic sulfur compounds cysteine and glutathionine [13]. Hydrogen sulfide is released from the cell as result of the sulfate reduction sequence pathway [1,13]. Hydrogen sulfide is derived from the HS- ion, which serves as an intermediate stage in the reduction of sulfite and sulfate to synthesize organic sulfur compounds[1,13]. The HS- ion is sequestered in the presence of a nitrogen supply to form methionine and cysteine. Thus in low nitrogen environments, sulfide gathers within the cell and be released as hydrogen sulfide [1,13]. This can cause off flavor in the wine. | ||
==Yeast Assimilable Nitrogen and Free Amino Nitrogen== | ==Yeast Assimilable Nitrogen and Free Amino Nitrogen== | ||
Yeast assimilable nitrogen (YAN) is important for any beverage fermentation process. YAN refers to the nitrogenous compounds like | ===Why Nitrogen?=== | ||
Nitrogen is considered a building block of life necessary to the growth of almost any organism. It is part of many biomolecules and molecules inherent to cell machinery. It is necessary for both the synthesis of Acetyl-CoA and amino acids, which yeast cells need for proliferation [2]. It also makes nitrogen a convenient measure to take when planning for fermentation, according to multiple agricultural research websites, though the articles are not always peer reviewed. The same tutorial style information explains how nitrogen levels in the must and juice can indicate how long a fermentation might take. Furthermore, low nitrogen levels are associated with what are known as stuck fermentations, where yeast growth declines [1,2,14]. Thus YAN is an important measurement for the complete fermentation and preservation of flavor and aroma in wine. | |||
YAN is widely accepted as an important measurement during wine making. Typical YAN levels vary between grape type, climate, harvest, and fruit health. YAN should be measured at the start of most large quantity fermentations, due to its inherent impact on the fermentation process, as explained below [see website http://www.vtwines.info/]. Certain problems in grape growth and development like rot deplete YAN [1,2,14, VT wine website]. Nitrogen availability is important because of its relation to the formation sulfur-like compounds. Sulfur like compounds are recognized compounds found in wines that produce off, or unpalatable flavors [1,2,14]. The necessary YAN for fermentation is very relative to varietal and geography, so across the US universities like Virginia Tech measure YAN in local wineries. | |||
One particular aspect of wine making includes overcoming the variability of grape juice from year to year. Depending on the season and climate, grapes can have different sugar, amino acid, and nitrogen compositions at harvest time. The interplay between these and other major compounds is what winemakers attempt to fine tune in order to create palatable vintages year after year, in the context of changing grape conditions. | |||
Yeast assimilable nitrogen (YAN) is important for any beverage fermentation process. YAN refers to the nitrogenous compounds like Free Amino Nitrogen (FAN), ammonia, and ammonium available in grapes. The most important sources of nitrogenous compounds are alpha amino acids, ammonium ion concentrations and small peptides, excluding Proline a dominant secondary amino acid [1]. | |||
Agricultural research institutes across the world track YAN levels as they relate to cultivars. In Australia the addition of YAN is common practice in many large-scale operations. Among Australian grape juices YAN can vary from 50-350 mg/L [1,6]. Yeast also varies in its metabolic demand for nitrogen. Besides fermentable sugars, nitrogen availability is the primary nutrient in yeast fermentation [2]. YAN can be measured after harvest, depending on the level of facilities available. Controlling YAN is critical to all wine making to ensure spoilage organisms don’t ruin the wines sensory quality [1,6] | |||
Lack of YAN can be the cause of things like stuck fermentations, where yeast stop metabolizing and enter a dormant state. Diammonium phosphate can be added as a nutrient to correct for YAN levels, often at the beginning of the fermentation process [1,6]. | |||
===Free Amino Nitrogen=== | |||
Free amino nitrogen is assimilable, or available, to yeast for metabolic activity during the fermentation process. Thus because nitrogen availability is so strongly tied good fermentation, the amount of FAN is of interest to enologists. FAN is determined by assaying the alpha amino group of primary amino acids, or FAN. Proline is exluded from the assay because though it contains FAN, the nitrogen in Proline is not assimilable in aerobic fermentation environments [2]. The measurement of non-Proline FAN and combined with the measurement of ammonia by enzymatic method is a sophisticated method for measuring YAN [see VT website mentioned above]. Simpler methods for measuring YAN include the Formal Titration, and more complex ones like mid-infrared spectrometry [1]. | |||
Free amino nitrogen is assimilable, or available, to yeast for metabolic activity during the fermentation process. | Many different wine compounds derived from fermentation are regulated by YAN. These compounds vary due to YAN levels and yeast species. Primary metabolites of sugar metabolism are controlled by YAN levels. Ethanol is another major product of sugar fermentation but generally, YAN has little effect on the ethanol yield. Glycerol and acetic acid are strongly dependent on both initial YAN levels, and yeast strain used. Sulfur dioxide production is also stimulated by initial YAN concentrations, but is shown to be largely yeast species dependent. Malolactic fermentation inhibition is also associated with high YAN levels, but not SO2 production [ | ||
Many different wine compounds derived from fermentation are regulated by YAN. | |||
===Future YAN applications & methods=== | ===Future YAN applications & methods=== | ||
Researchers have begun to assess ways to best control YAN in the industrial winemaking process. Yeast is shown to produce key aroma compounds, esters, alcohols, acids and lactones. One study examined two yeast strains that created different wines under initial nitrogen conditions to test the common practice of adding YAN before identifying levels of naturally occurring YAN. | Researchers have begun to assess ways to best control YAN in the industrial winemaking process. Yeast is shown to produce key aroma compounds, esters, alcohols, acids and lactones. One study examined two yeast strains that created different wines under initial nitrogen conditions to test the common practice of adding YAN before identifying levels of naturally occurring YAN. | ||
== | ==Conclusion== | ||
Wine is a complex beverage made up of many different biomolecules that stimulate the senses, particularly taste and smell. The production of wine is a large industry and research enables changes in practical applications to enhance flavor and aroma. This article covered just some of the compounds responsible for flavor and aroma, and how they relate to metabolic processes during the fermentation process. There is obviously much more to know about how these systems work, but the basic information is provided. Last the article explains how certain management strategies, related to microbial environments in the wine, can be managed to select for beneficial flavor compounds, and minimize off compounds. | |||
==References== | ==References== | ||
[ | [1] [http://link.springer.com/article/10.1007/s10295-011-1018-4 Styger, Gustav, Bernard Prior, and Florian F. Bauer. "Wine flavor and aroma." Journal of industrial microbiology & biotechnology 38.9 (2011): 1145-1159.] | ||
[ | [2] Slonczewski, Joan L., and John Watkins. Foster. Microbiology: An Evolving Science. New York: W.W. Norton Et, 2014. Print. | ||
[3] [http://onlinelibrary.wiley.com/doi/10.1111/j.1755-0238.2005.tb00285.x/abstract Swiegers, J. H., et al. "Yeast and bacterial modulation of wine aroma and flavour." Australian Journal of grape and wine research 11.2 (2005): 139-173.] | |||
[http://aem.asm.org/content/48/5/1034.short Heard, Gillian M., and Graham H. Fleet. "Growth of natural yeast flora during the fermentation of inoculated wines." Applied and Environmental Microbiology 50.3 (1985): 727-728.] | [http://aem.asm.org/content/48/5/1034.short Heard, Gillian M., and Graham H. Fleet. "Growth of natural yeast flora during the fermentation of inoculated wines." Applied and Environmental Microbiology 50.3 (1985): 727-728.] | ||
[http://www.sciencedirect.com/science/article/pii/S0168160503002903 Romano, P., et al. "Function of yeast species and strains in wine flavour." International journal of food microbiology 86.1 (2003): 169-180.] | [4] [http://www.sciencedirect.com/science/article/pii/S0168160503002903 Romano, P., et al. "Function of yeast species and strains in wine flavour." International journal of food microbiology 86.1 (2003): 169-180.] | ||
[ | [5] [http://aem.asm.org/content/65/2/740.full Nielsen, Jan Clair, and Marianne Richelieu. "Control of flavor development in wine during and after malolactic fermentation by Oenococcus oeni." Applied and environmental microbiology 65.2 (1999): 740-745.] | ||
[http://onlinelibrary.wiley.com/doi/10.1111/j.1755-0238.2005.tb00285.x/abstract Swiegers, J. H., et al. "Yeast and bacterial modulation of wine aroma and flavour." Australian Journal of grape and wine research 11.2 (2005): 139-173.] | [6] [http://onlinelibrary.wiley.com/doi/10.1111/j.1755-0238.2005.tb00285.x/abstract Swiegers, J. H., et al. "Yeast and bacterial modulation of wine aroma and flavour." Australian Journal of grape and wine research 11.2 (2005): 139-173.] | ||
[ | [7] [http://www.ncbi.nlm.nih.gov/pubmed/10532386 Lonvaud-Funel, Aline. "Lactic acid bacteria in the quality improvement and depreciation of wine." Lactic Acid Bacteria: Genetics, Metabolism and Applications. Springer Netherlands, 1999. 317-331.] | ||
[http:// | [8] [http://www.lodigrowers.com/download/wine_quality/Sinton_IndicatorWineQuality_1978.pdf T. H., et al. "Grape juice indicators for prediction of potential wine quality. I. Relationship between crop level, juice and wine composition, and wine sensory ratings and scores." American Journal of Enology and Viticulture 29.4 (1978): 267-271.] | ||
[http://pubs.acs.org/doi/pdf/10.1021/jf0342019 Ugliano, Maurizio, Alessandro Genovese, and Luigi Moio. "Hydrolysis of wine aroma precursors during malolactic fermentation with four commercial starter cultures of Oenococcus oeni." Journal of agricultural and food chemistry 51.17 (2003): 5073-5078.] | [9] [http://pubs.acs.org/doi/pdf/10.1021/jf0342019 Ugliano, Maurizio, Alessandro Genovese, and Luigi Moio. "Hydrolysis of wine aroma precursors during malolactic fermentation with four commercial starter cultures of Oenococcus oeni." Journal of agricultural and food chemistry 51.17 (2003): 5073-5078.] | ||
[http:// | [10] [http://ry6af4uu9w.scholar.serialssolutions.com/?sid=google&auinit=MA&aulast=Sefton&atitle=Compounds+causing+cork+taint+and+the+factors+affecting+their+transfer+from+natural+cork+closures+to+wine%E2%80%93a+review&id=doi:10.1111/j.1755-0238.2005.tb00290.x&title=Australian+journal+of+grape+and+wine+research&volume=11&issue=2&date=2005&spage=226&issn=1322-7130 Sefton, Mark A., and Robert F. Simpson. "Compounds causing cork taint and the factors affecting their transfer from natural cork closures to wine–a review." Australian Journal of Grape and Wine Research 11.2 (2005): 226-240.] | ||
[http:// | [11] [http://wsldocs.sos.wa.gov/library/docs/wsu/extension/xb1026e_2008_004880.pdf Edwards, Charles Gould. Lactic acid bacteria native to Washington State wines. Washington State University, College of Agriculture and Home Economics Research Center, 1992.] | ||
[http:// | [12] [http://informahealthcare.com/doi/abs/10.3109/10408418609108739?journalCode=mby Casey, Gregory P., and WM Mike Ingledew. "Ethanol tolerance in yeasts." Critical reviews in microbiology 13.3 (1986): 219-280.] | ||
[13] [http://www.ncbi.nlm.nih.gov/pubmed/17262212 Swiegers, J. H., and I. S. Pretorius. "Modulation of volatile sulfur compounds by wine yeast." Applied Microbiology and Biotechnology 74.5 (2007): 954-960.] | |||
[14] [http://onlinelibrary.wiley.com/doi/10.1111/j.1567-1364.2008.00412.x/full Carrau, Francisco M., et al. "Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: effects of yeast assimilable nitrogen on two model strains." FEMS yeast research 8.7 (2008): 1196-1207.] | |||
[15] [www.ftb.com.hrfiles/journals/1/articles/183/public/183-182-1-PB.pdf Romano, Patrizia. "Metabolic characteristics of wine strains during spontaneous and inoculated fermentation." Food Technology and Biotechnology 35 (1997): 255-26] | |||
<!--Do not remove this line--> | <!--Do not remove this line--> | ||
Latest revision as of 05:59, 16 April 2015
Wine making or vinification is a process through which juice, typically grape, is fermented into an alcoholic beverage. Vinification is a complex multistep process, with many uncontrollable variables to be accounted for [1,2,3,4]. Vinification requires the careful management of the microflora present, nutrient availability for the microflora, pH, temperature, and storage conditions, in order to make a palatable vintage. Viticulture refers to the manage the soil, vines, fruit health and harvest, while enology refers to all other aspects of the process that turn the juice to wine. Viticulture and enology research support a demand within the wine industry to develop scientifically supported practices for managing problems in the field. Varietal refers to the grape type, such as a Chardonnay or Petite Sirah, and each varietal is endemic to different climates. Ultimately though, due to the value of a palatable wine, the science of vinification is most used to improve the flavor and aroma of the final product.
The perception of a wine’s flavor and aroma comes from a multitude of interactions between about a 1,000 chemical compounds within the wine and our own sensory receptors [1,3,8]. These chemical compounds function in many different ways, and can act synergistically or antagonistically with each other [1,3]. Flavor and aroma compounds are derived from the grapes themselves, the wood fermentation occurs in, and the metabolites of microbes used in the fermentation process [1,2,3]. Making a tasty wine is largely related to the ratios of volatile compounds found in the wine; wines without enough of a certain compound will lack flavor and fullness, while wines with too much of a compound can taste spoiled [1,2,3,4,5,8]. These compounds result from both the alcoholic fermentation process and the malolactic fermentation, which are each driven by their own microbes [2].
Biological and chemical principles guide the science behind the entire vinification process. Once fruit is harvested, it is the winemakers duty to ferment the juice into a palatable wine through complex interactions of yeast and bacteria [1,2,8]. Fermentation in wine is achieved primarily by the yeast Saccharomyces cerevisiae and secondarily through fermentation by malolactic bacteria [1,2,5-7]. Grapes in the field, and in vats after harvest contain natural amounts of residual nutrients, bacteria, and yeast [1,2]. The grape must is a combination of juice, seeds, skins, and stems, along with a host of microbes. Upon crushing the grapes, the juice is naturally inoculated with the yeasts and bacteria from the must. Common industry practice dictates inoculating juice with yeast from a cultured stock bred for vinification to dominate the fermentation process in a favorable way [2]. To determine the length of the fermentation, along with what nutrients to add, enologist assess the biochemical state of the wine throughout fermentation [1,2,3,8] . Concern is paid to particular bacteria, and yeast strains associated with spoilage. Spoilage refers to the presence of compounds associated with off putting aroma and flavor and is explained in more depth in section 2. More important than identifying problematic microbes, is thorough management of the microbial environment of the wine. The proper microbial environment during fermentation ensures that the intended organisms thrive and outcompete spoilage microbes [2]. This includes measuring pH, sugar (or brix), Yeast assimilable nitrogen (YAN), and Free Amino Nitrogen (FAN) discussed in section three, which all help manage spoilage [1,2,3,8].
The page is aimed at a general overview of vinification practices and research into these practices that deal with flavor and aroma. That includes a brief overview of the microbial activity in vinification (alcohol and malolactic) to understand some of the general organisms involved in the process. One the basic fermentation process is outlined in a biological context, it is important to what compounds in wine are important to flavor and aroma, and how the compounds result from microbial metabolism. This includes covering flavor modulation by yeast, and the formation of hydrogen sulfide (spoilage) compounds. Last, common practical applications for altering the flavor compounds or microbial processes in wine are discussed.
Microbial Processes of Vinification
Alcoholic Fermentation
Alcohol fermentation is the metabolic process through which sugars are converted to ethanol and carbon dioxide (Micro text). Ripe grapes contain high levels of the simple sugars fructose and glucose (~15%) which are quickly metabolized into pyruvate by yeast [2]. Pyruvate is then used in the biosynthesis of essential constituents like amino acids, and fatty acids, with by products of carbon dioxide, diacetyl, ethanol, esters, and other long chain alcohols [2]. See the figures for additional guidance in the process. Figure two has a description of the fundamental chemical changes in fermentation, figure three shows a chart of the major chemicals produced in wine fermentation. It is these chemicals along with variations in other flavor compounds formed during vinification that make make up the sensory quality of a wine [3,8]. Yeast, mainly Saccharomyces cerevisiae can ferment in aerobic and anaerobic conditions, and is the main contributor to alcohol formation because of its tolerance to high ethanol concentrations (ethanol tolerance in yeast 1986). Other yeasts naturally found in the grape must include Kloeckera and Hanseniaspora [1,2]. S. cerevisiae is shown to modify lipids structures to endure the stress of an ethanol environment [9]. Typically the inoculated yeast populations dominate the later stages of fermentation, a period lasting about 6-20 days depending on initial sugar concentration, or until ethanol reaches about 15% and yeast growth stops [1,2].
Malolactic Fermentation
The secondary phase of wine fermentation is known as malolactic fermentation. It occurs in all red wines, and some whites. After fermentation, the wine is drained from the sediment and yeast, and put into various containers. This is the point in the fermentation process in which wood might be introduced in the form of a wooden barrel, while other winemakers select stainless steel depending on the desired taste [1,2,5-7]. In some cases, wine is fermented a second time by malolactic fermentation. Malolactic fermentation is the decarboxylation of L-malic acid to L-lactic acid through enzymatic activity [2]. This chemical process decreases the acidity from the malic acid in grapes by converting malic acid to L-malate which is fermented by Oenococcus oeni [1,2,5]. See figure four for a illustration of this chemical process. Several lactic acid bacteria can be responsible for malolactic fermentation, with varying effects on the wines flavor and aroma. Lactic acid bacteria in grape must come from the genera Lactobacillus, Pediococcus, Leuconostoc, and Oenococcus. Citric and malic acid can also be fermented by the lactic acid bacteria known as Oenococcus oeni [5,6,7]. The bacteria can be bought to be added manually, but they also occur naturally. The process of citric acid fermentation produces diacetyl, a compound associated with nutty and buttery aromas in wine [2,5]. Sulfite is commonly added to the wine after malolactic fermentation to stop microbial activity, which may prohibit the wine from completing the degradation of citric acid [2,5]. The bacteria can contribute to aroma and flavor of wine by the process of malolactic fermentation where grape derived secondary metabolites can be hydrolyze glycosylated aroma precursors [9].
Certain spoilage bacteria give wine off flavors and poor aroma [1,2,8]. These can be bacteria or molds that grow within in wine corks [10]. Growth of the bacteria Pediococcus spp can have negative impacts on wine flavor and aroma [1,2,5-7]. An example is the formation of diacetyl and acetoin. Growth of the Lactobacillus spp can also have negative impacts on flavor and aroma. Uncontrolled growth of the species is associated with volatile acidity and the formation of compounds like diacetyl, acetoin, and Heresztyn [11]. Sulfur Dioxide is commonly used to control the growth of the aforementioned undesirable bacteria [1,2]. Sulfur dioxide and the pH of win inhibit Lactobacillus spp growth [2,5,6,11]. Though some Pediococcus and Lactobacillus contribute to favorable flavor characteristics, excessive growth of the bacteria will cause spoiling [11].
Compounds That Effect Flavor and Aroma
Wine aroma and flavor is caused by the presence of various compounds intrinsic to the climate and fermentation process. The low pH and high sugar content of grapes exerts a strong selective regime on microorganism within the wine, so that only certain yeast and bacteria species are able to thrive [2,8,12]. The selection regime for a fermenting must only strengthens in anaerobic conditions. The roles individual bacteria and yeast species play in fermentation are directly tied to the taste and smell of the wine as a final product [1,3,4]. The following section outlines in depth the flavor and aroma compounds that give a wine its complexion, and describes how briefly how they are synthesized. Many flavor and aroma compounds contribute beneficially to a wine at some level, but at to high concentration they produce an off flavor. Some of the main compounds and how they relate to fermentation are described in figure 4.
Flavor modulation by yeast
The following 16 yeast species are common in wine making process: Brettanomyces, Dekkera, Candida, Cryptococcus, Debaryomyces, Hanseniaspora and its asexual counterpart Kloeckera, Kluyveromyces, Metschnikowia, Pichia, Rhodotorula, Saccharomyces, Saccharomyces, Schizosaccharomyces, Torulaspora and Zygosaccharomyces [1]. Throughout the fermentation process different yeast thrive or dwindle according to nutrient availability and environmental conditions. Kloeckera, Hanseniaspora and Candida are shown to thrive in early stages. Several species of Metschnikowia and Pichia follow as concentrations rise. During the latter stages of fermentation in higher alcohol concentrations, Saccharomyces cerevisiae thrives [1,3].
Non-volatile acids
Non volatile acids influences the growth of microbes, and the overall chemistry of the wine. It is responsible for characteristics like ‘freshness’ among certain varietals. The acidity of grapes is dependent on the temperature of the region [1,2,4]. Some acid species found in wine during the fermentation process include succinic acid, keto acids (pyruvic and ketoglutaric acid), lactic acid, and malic acid. Succinic acid, associated with yeast fermentation, is known to have a bitter and overly salty taste [1,2].
Volatile organic acids
Volatile organic acids are also major in wine chemistry. Among the volatile acid species are acetic acid (90%) and the rest comprised of propionic and hexanoic acid. They result from fatty acid metabolism in yeast and bacteria. Acetic acid can impart a vinegar taste at high concentrations. Acetate is produced by yeast, but most acetic acid in wine comes from the metabolism of ethanol by aerobic acetic acid bacteria [1,2]. Alcohols – ethanol is the main alcohol of interest in wine. High sugar levels in fruit convert to wines with more ethanol. Enough ethanol enhances the sensory perception of a wine, but too much produces a burn that can mask the wine [1,2,3,4].
Glycerol
Glycerol is another product of the alcoholic fermentation process. Glycerol is a polyol that tasts slightly sweet with an oily mouth feel. Glycerol is found in dry and semi-sweet wines at concentrations of 5-14 g/L [1]. In red wines glycerol concentrations are higher than in whites. Glycerol metabolism is very important to the anaerobic fermentation of sugars because it plays key roles in cell growth and division [1].
Higher alcohols or fusel alcohols are secondary yeast metabolites. Higher alcohols can have beneficial or detrimental effects on wine flavor and aroma. Too much of a higher alcohol gives wine a strong pungent smell, but the right amount contributes to a wines fruit notes [1,2,3]. Higher alcohol formation can be variable among different yeast strains. Yeast products include acetaldehyde, pyruvic acid, and vinyl phenol [1,2,3].
Another compound important to taste is diacetyl which in smaller concentrations gives wine a buttery or toasty flavor, but at high concentrations it is off putting. Most diacetyl in wine comes from the metabolic activities of lactic acid bacteria [1,2]. Other compound varieties include volatile phenols, esters, sulfur compounds, sulfides, thiols and monoterpenoids [1,2,4].
Hydrogen Sulfide
Yeast has role in production of volatile sulfur compounds through the sulfate reduction sequence pathway [1,2,13]. The importance of sulfur compounds in wine has a tremendous effect on the sensory quality of the wine. Hydrogen sulfide can make wines smell rotten while other sulfur compounds like the flavor active thiol 3-mercaptohexanol, can impart fruity notes beneficial to the sensory quality of a wine [13]. The wine yeast Saccharomyces cerevisiae is responsible for forming many of these volatile sulfur compounds that contribute to a wines sensory quality [1,13].
Hydrogen sulfides found in wine are formed through the metabolic pathways of yeast from inorganic sulfur compounds, sulfate and sulfite, and from organic sulfur compounds cysteine and glutathionine [13]. Hydrogen sulfide is released from the cell as result of the sulfate reduction sequence pathway [1,13]. Hydrogen sulfide is derived from the HS- ion, which serves as an intermediate stage in the reduction of sulfite and sulfate to synthesize organic sulfur compounds[1,13]. The HS- ion is sequestered in the presence of a nitrogen supply to form methionine and cysteine. Thus in low nitrogen environments, sulfide gathers within the cell and be released as hydrogen sulfide [1,13]. This can cause off flavor in the wine.
Yeast Assimilable Nitrogen and Free Amino Nitrogen
Why Nitrogen?
Nitrogen is considered a building block of life necessary to the growth of almost any organism. It is part of many biomolecules and molecules inherent to cell machinery. It is necessary for both the synthesis of Acetyl-CoA and amino acids, which yeast cells need for proliferation [2]. It also makes nitrogen a convenient measure to take when planning for fermentation, according to multiple agricultural research websites, though the articles are not always peer reviewed. The same tutorial style information explains how nitrogen levels in the must and juice can indicate how long a fermentation might take. Furthermore, low nitrogen levels are associated with what are known as stuck fermentations, where yeast growth declines [1,2,14]. Thus YAN is an important measurement for the complete fermentation and preservation of flavor and aroma in wine.
YAN is widely accepted as an important measurement during wine making. Typical YAN levels vary between grape type, climate, harvest, and fruit health. YAN should be measured at the start of most large quantity fermentations, due to its inherent impact on the fermentation process, as explained below [see website http://www.vtwines.info/]. Certain problems in grape growth and development like rot deplete YAN [1,2,14, VT wine website]. Nitrogen availability is important because of its relation to the formation sulfur-like compounds. Sulfur like compounds are recognized compounds found in wines that produce off, or unpalatable flavors [1,2,14]. The necessary YAN for fermentation is very relative to varietal and geography, so across the US universities like Virginia Tech measure YAN in local wineries.
One particular aspect of wine making includes overcoming the variability of grape juice from year to year. Depending on the season and climate, grapes can have different sugar, amino acid, and nitrogen compositions at harvest time. The interplay between these and other major compounds is what winemakers attempt to fine tune in order to create palatable vintages year after year, in the context of changing grape conditions.
Yeast assimilable nitrogen (YAN) is important for any beverage fermentation process. YAN refers to the nitrogenous compounds like Free Amino Nitrogen (FAN), ammonia, and ammonium available in grapes. The most important sources of nitrogenous compounds are alpha amino acids, ammonium ion concentrations and small peptides, excluding Proline a dominant secondary amino acid [1].
Agricultural research institutes across the world track YAN levels as they relate to cultivars. In Australia the addition of YAN is common practice in many large-scale operations. Among Australian grape juices YAN can vary from 50-350 mg/L [1,6]. Yeast also varies in its metabolic demand for nitrogen. Besides fermentable sugars, nitrogen availability is the primary nutrient in yeast fermentation [2]. YAN can be measured after harvest, depending on the level of facilities available. Controlling YAN is critical to all wine making to ensure spoilage organisms don’t ruin the wines sensory quality [1,6]
Lack of YAN can be the cause of things like stuck fermentations, where yeast stop metabolizing and enter a dormant state. Diammonium phosphate can be added as a nutrient to correct for YAN levels, often at the beginning of the fermentation process [1,6].
Free Amino Nitrogen
Free amino nitrogen is assimilable, or available, to yeast for metabolic activity during the fermentation process. Thus because nitrogen availability is so strongly tied good fermentation, the amount of FAN is of interest to enologists. FAN is determined by assaying the alpha amino group of primary amino acids, or FAN. Proline is exluded from the assay because though it contains FAN, the nitrogen in Proline is not assimilable in aerobic fermentation environments [2]. The measurement of non-Proline FAN and combined with the measurement of ammonia by enzymatic method is a sophisticated method for measuring YAN [see VT website mentioned above]. Simpler methods for measuring YAN include the Formal Titration, and more complex ones like mid-infrared spectrometry [1].
Many different wine compounds derived from fermentation are regulated by YAN. These compounds vary due to YAN levels and yeast species. Primary metabolites of sugar metabolism are controlled by YAN levels. Ethanol is another major product of sugar fermentation but generally, YAN has little effect on the ethanol yield. Glycerol and acetic acid are strongly dependent on both initial YAN levels, and yeast strain used. Sulfur dioxide production is also stimulated by initial YAN concentrations, but is shown to be largely yeast species dependent. Malolactic fermentation inhibition is also associated with high YAN levels, but not SO2 production [
Future YAN applications & methods
Researchers have begun to assess ways to best control YAN in the industrial winemaking process. Yeast is shown to produce key aroma compounds, esters, alcohols, acids and lactones. One study examined two yeast strains that created different wines under initial nitrogen conditions to test the common practice of adding YAN before identifying levels of naturally occurring YAN.
Conclusion
Wine is a complex beverage made up of many different biomolecules that stimulate the senses, particularly taste and smell. The production of wine is a large industry and research enables changes in practical applications to enhance flavor and aroma. This article covered just some of the compounds responsible for flavor and aroma, and how they relate to metabolic processes during the fermentation process. There is obviously much more to know about how these systems work, but the basic information is provided. Last the article explains how certain management strategies, related to microbial environments in the wine, can be managed to select for beneficial flavor compounds, and minimize off compounds.
References
[2] Slonczewski, Joan L., and John Watkins. Foster. Microbiology: An Evolving Science. New York: W.W. Norton Et, 2014. Print.
[3] Swiegers, J. H., et al. "Yeast and bacterial modulation of wine aroma and flavour." Australian Journal of grape and wine research 11.2 (2005): 139-173. Heard, Gillian M., and Graham H. Fleet. "Growth of natural yeast flora during the fermentation of inoculated wines." Applied and Environmental Microbiology 50.3 (1985): 727-728.
[15] [www.ftb.com.hrfiles/journals/1/articles/183/public/183-182-1-PB.pdf Romano, Patrizia. "Metabolic characteristics of wine strains during spontaneous and inoculated fermentation." Food Technology and Biotechnology 35 (1997): 255-26]
Edited by Patrick Niedermeyer, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.