Cryptosporidium: Difference between revisions
No edit summary |
No edit summary |
||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{ | {{Curated}} | ||
{{Biorealm Genus}} | |||
{ | |||
[[Image:CryptosporidiumParvum.jpg | [[Image:CryptosporidiumParvum.jpg|thumb|300px|right|''Cryptosporidium parvum.'' Image from [http://www.cbu.edu/%7Eseisen/CadSu0402.htm Caduceus Newsletter, July 2004.]]] | ||
==Classification== | ==Classification== | ||
===Higher order taxa:=== | |||
Eukaryota; Alveolata; Apicomplexa; Coccidia; Eimeriida; Cryptosporidiidae | |||
===Species:=== | |||
''Cryptosporidium parvum, C. andersoni, C. baileyi '' | |||
'' | {| | ||
| height="10" bgcolor="#FFDF95" | | |||
'''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Tree&id=5806&lvl=3&lin=f&keep=1&srchmode=1&unlock Taxonomy] Genome''' | |||
|} | |||
==Description and Significance== | ==Description and Significance== | ||
''Cryptosporidium'' is a genus of parasites which has become a rising concern due to its presence in drinking water. The species that affects the most mammals, including humans, is ''Cryptosporidium parvum'', which may cause gastrointestinal illness. In individuals with healthy immune systems the disease may lead to watery diarrhea for up to several weeks, but in those with compromised immune systems the pathogen may be prolonged and even life-threatening. As of yet there is no known cure for this illness, although antibiotics have proved somewhat effective in curbing the immediate symptoms. This fact and recent suspected outbreaks have led to an increased interest in methods to prevent people and animals from coming into contact with ''Cryptosporidium'' are underway, especially in regards to water supply regulation. Young children, people with compromised immune systems, international travelers, and people who drink from or swim in shallow or unprotected water are most at risk for this parasite. As in the case of similar parasites, ''Cryptosporidium'' gained prevalence during the AIDS epidemic, and gained importance as fatalies grew among AIDS patients with crytosporidiosis. | |||
== | ==Genome Structure== | ||
Thus far eight chromosomes of ''Cryptosporidium'' have been sequenced, ranging in size from 900-13000 kb. As this is an emerging pathogen, projects are currently underway to better understand the parasitic species' genetics and relationships to each other. | |||
==Cell Structure and Metabolism== | ==Cell Structure and Metabolism== | ||
[[Image:cry1b.jpg|thumb|300px|left|Oocysts of ''Cryptosporidium parvum.'' Image from [http://www.cdfound.to.it/HTML/khan.htm O.A. Khan.]]] | |||
The life cycle of ''Cryptosporidium'' begins as the host ingests the parasite in its infective stage, the oocyst. Inside the host the oocyst releases four sporozoites, which move into the intestine and take up residence within the host's epithelial cells. Two asexual cycles take place, resulting in the production of meronts. First generation meronts contain eight merozoites; second generation meronts contain four merozoites. This second generation of merozoites invade other cells and develop into either female or male gametes. The male gametes break out of the host cells and fertilize female gametes in other cells. The fertilized female gametes morph into oocysts and in turn break out of the host cells. Two kinds of oocysts are produced: a thin-walled type which stays inside the host and continues replication and infection, and a thick-walled type which is excreted in the host's fecal matter. During these processes the host cells are destroyed, putting the immune system into action and causing many of the clinical symptoms of gastrointestinal disease. The oocysts are generally small spheres, 4-5 micrometers in diameter, and are highly resilient against chemicals and outside environment factors. | The life cycle of ''Cryptosporidium'' begins as the host ingests the parasite in its infective stage, the oocyst. Inside the host the oocyst releases four sporozoites, which move into the intestine and take up residence within the host's epithelial cells. Two asexual cycles take place, resulting in the production of meronts. First generation meronts contain eight merozoites; second generation meronts contain four merozoites. This second generation of merozoites invade other cells and develop into either female or male gametes. The male gametes break out of the host cells and fertilize female gametes in other cells. The fertilized female gametes morph into oocysts and in turn break out of the host cells. Two kinds of oocysts are produced: a thin-walled type which stays inside the host and continues replication and infection, and a thick-walled type which is excreted in the host's fecal matter. During these processes the host cells are destroyed, putting the immune system into action and causing many of the clinical symptoms of gastrointestinal disease. The oocysts are generally small spheres, 4-5 micrometers in diameter, and are highly resilient against chemicals and outside environment factors. | ||
==Ecology | ==Ecology== | ||
[[Image:cryptosporidium-parvum3.jpg|thumb|300px|right|''Cryptosporidium parvum.'' Image from [http://www.waterfilterreview.com/info_h2o/contaminants/water_contaminant_list.html www.WaterFilterReview.com]]] | |||
As ''Cryptosporidium'' is primarily contracted through drinking water, the prevention and control of this parasite has become a public health concern. Various government committees and associations are at work to regulate the decontamination of both public and private water sources. Prevention methods include the installation of more advanced filtration systems, constant testing of water supply, and educating populations about the signs of the disease and ways to prevent it. The distribution of ''Cryptosporidium'' is worldwide, but outbreaks are especially common in developing countries and those with high rates of AIDS among the population. | |||
==References== | |||
[http://www.nal.usda.gov/wqic/cryptfac.html Atwill, Rob. ''Cryptosporidium parvum ''and Cattle: Implications for Public Health and Land Use Restrictions. Medical Ecology and Environmental Animal Health, University of California-Davis.] | |||
[http://www.lpsi.barc.usda.gov/awpl/cryptosporidium.asp ''Cryptosporidium.'' Animal Waste Pathogen Laboratory, U.S. Department of Agriculture.] | |||
[http://www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_cryptosporidiosis.htm ''Cryptosporidium'' Infection Fact Sheet. Division of Parasitic Diseases, Centers for Disease Control and Prevention.] | |||
[http://www.cdfound.to.it/HTML/khan.htm Khan, Omar A. ''Cryptosporidium parvum.'' Atlas of Medical Parasitology, Carlo Denegri Foundation.] | |||
[http://www.ksu.edu/parasitology/research Upton, Steve J. ''Cryptosporidium'' Research. Division of Biology, Kansas State University]. | |||
[http://www.awwa.org/Advocacy/govtaff/whawapap.cfm What Water Utilities Can Do to Minimize Public Exposure to ''Cryptosporidium'' in Drinking Water. Government Affairs, American Water Works Association. 2005.] | |||
Latest revision as of 15:01, 7 August 2010
A Microbial Biorealm page on the genus Cryptosporidium
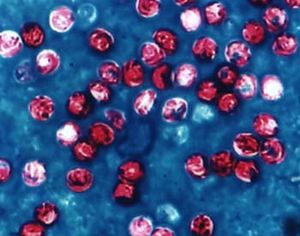
Classification
Higher order taxa:
Eukaryota; Alveolata; Apicomplexa; Coccidia; Eimeriida; Cryptosporidiidae
Species:
Cryptosporidium parvum, C. andersoni, C. baileyi
|
NCBI: Taxonomy Genome |
Description and Significance
Cryptosporidium is a genus of parasites which has become a rising concern due to its presence in drinking water. The species that affects the most mammals, including humans, is Cryptosporidium parvum, which may cause gastrointestinal illness. In individuals with healthy immune systems the disease may lead to watery diarrhea for up to several weeks, but in those with compromised immune systems the pathogen may be prolonged and even life-threatening. As of yet there is no known cure for this illness, although antibiotics have proved somewhat effective in curbing the immediate symptoms. This fact and recent suspected outbreaks have led to an increased interest in methods to prevent people and animals from coming into contact with Cryptosporidium are underway, especially in regards to water supply regulation. Young children, people with compromised immune systems, international travelers, and people who drink from or swim in shallow or unprotected water are most at risk for this parasite. As in the case of similar parasites, Cryptosporidium gained prevalence during the AIDS epidemic, and gained importance as fatalies grew among AIDS patients with crytosporidiosis.
Genome Structure
Thus far eight chromosomes of Cryptosporidium have been sequenced, ranging in size from 900-13000 kb. As this is an emerging pathogen, projects are currently underway to better understand the parasitic species' genetics and relationships to each other.
Cell Structure and Metabolism
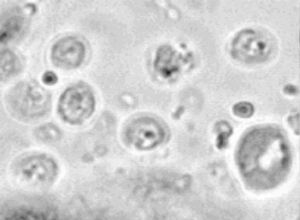
The life cycle of Cryptosporidium begins as the host ingests the parasite in its infective stage, the oocyst. Inside the host the oocyst releases four sporozoites, which move into the intestine and take up residence within the host's epithelial cells. Two asexual cycles take place, resulting in the production of meronts. First generation meronts contain eight merozoites; second generation meronts contain four merozoites. This second generation of merozoites invade other cells and develop into either female or male gametes. The male gametes break out of the host cells and fertilize female gametes in other cells. The fertilized female gametes morph into oocysts and in turn break out of the host cells. Two kinds of oocysts are produced: a thin-walled type which stays inside the host and continues replication and infection, and a thick-walled type which is excreted in the host's fecal matter. During these processes the host cells are destroyed, putting the immune system into action and causing many of the clinical symptoms of gastrointestinal disease. The oocysts are generally small spheres, 4-5 micrometers in diameter, and are highly resilient against chemicals and outside environment factors.
Ecology

As Cryptosporidium is primarily contracted through drinking water, the prevention and control of this parasite has become a public health concern. Various government committees and associations are at work to regulate the decontamination of both public and private water sources. Prevention methods include the installation of more advanced filtration systems, constant testing of water supply, and educating populations about the signs of the disease and ways to prevent it. The distribution of Cryptosporidium is worldwide, but outbreaks are especially common in developing countries and those with high rates of AIDS among the population.
References
Cryptosporidium. Animal Waste Pathogen Laboratory, U.S. Department of Agriculture.
Khan, Omar A. Cryptosporidium parvum. Atlas of Medical Parasitology, Carlo Denegri Foundation.
Upton, Steve J. Cryptosporidium Research. Division of Biology, Kansas State University.
