Chest Port Microbial Infections: Difference between revisions
| Line 5: | Line 5: | ||
A chest port is an indwelling catheter connected to a reservoir, inserted under the skin of the chest, and used to administer medicines directly into a vein over a long period of time (Figure 1). Physicians frequently utilize chest ports to administer multiple cycles of chemotherapy in children because of the ease of vascular access and care for port maintenance. Because chest ports are inserted under the skin, the skin covering the port acts as a barrier to potentially harmful microorganisms. In comparison to an IV line, chest ports can stay in place for months at a time, can be used to collect blood samples without needles, and have a lower risk of infection over time. Although chest port infections are not as common as other external catheter infections, the most significant complication during chemotherapeutic treatment via chest port catheters are microbial infections. Approximately 5% of patients diagnosed with a microbial port infection are treated by port excision.<ref>[Funaki, Brian. “Subcutaneous Chest Port Infection.” <b>(2005)</b>. <i>Seminars in Interventional Radiology</i>, 22.3: 245–247. <i>PMC</i>.] </ref> | A chest port is an indwelling catheter connected to a reservoir, inserted under the skin of the chest, and used to administer medicines directly into a vein over a long period of time (Figure 1). Physicians frequently utilize chest ports to administer multiple cycles of chemotherapy in children because of the ease of vascular access and care for port maintenance. Because chest ports are inserted under the skin, the skin covering the port acts as a barrier to potentially harmful microorganisms. In comparison to an IV line, chest ports can stay in place for months at a time, can be used to collect blood samples without needles, and have a lower risk of infection over time. Although chest port infections are not as common as other external catheter infections, the most significant complication during chemotherapeutic treatment via chest port catheters are microbial infections. Approximately 5% of patients diagnosed with a microbial port infection are treated by port excision.<ref>[Funaki, Brian. “Subcutaneous Chest Port Infection.” <b>(2005)</b>. <i>Seminars in Interventional Radiology</i>, 22.3: 245–247. <i>PMC</i>.] </ref> | ||
Infections of implanted devices most commonly result from <i>Staphylococcus epidermidis</i>, <i>Staphylococcus aureus</i>, <i>Enterococcus faecalis</i>, <i>Streptococcus vidrians</i>, <i>Klebsiella pneumonia</i>, and <i>Pseudomona aeruginosa</i>.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/24515846/ Paredes, J.,Alonso-Acre, M., Schmidt, C., Valderas, D., Sedano, B., Legarda, J., Arizti, F., Gomez, E., Aguinaga, A., Del Pozo, J.L., Arana, S. "Smart central venous port for early detection of bacterial biofilm related infections" <b>(2014)</b> <i>Biomed Microdevices</i>, 16: 365.]</ref> Of the above microbes, <i>S. epidermidis</i> is the most relevant port associated pathogen. Jukes et al. estimate in the United States, 80% of hospital acquired catheter related bloodstream infections (CRBSI) are caused by the microbe <i>S. epidermidis</i>.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/20813851/ Jukes, L., Mikhail, J., Bome-Mannathoko, N., Hadfield, S.J., Harris, L.G., El-Bouri, K., Davies, A.P., Mack, D. "Rapid differentiation of <i>Staphylococcus aureus</i>,<i>Staphylococcus epidermidis</i> and other coagulase-negative staphylococci and meticillin susceptibility testing directly from growth-positive blood cultures by multiplex real-time PCR" <b>(2010)</b> </i>J. Med. Microbiol</i>, 59:1456–1461]</ref> The treatment of these catheter-related bloodstream <i>S. epidermidis</i> infections costs the U.S. about $2 billion a year.<ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2807625/ Otto, Michael. “<i>Staphylococcus Epidermidis</i> – the ‘accidental’ Pathogen.” <b>(2009)</b> <i>Nature reviews. Microbiology</i> 7.8: 555–567. <i>PMC<b>.]</ref> Thus, research on practices that decrease chest port infection incidence and basic scientific research on the mechanism of chest infections can significantly reduce the problem of chest port infections. | Infections of implanted devices most commonly result from <i>Staphylococcus epidermidis</i>, <i>Staphylococcus aureus</i>, <i>Enterococcus faecalis</i>, <i>Streptococcus vidrians</i>, <i>Klebsiella pneumonia</i>, and <i>Pseudomona aeruginosa</i>.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/24515846/ Paredes, J.,Alonso-Acre, M., Schmidt, C., Valderas, D., Sedano, B., Legarda, J., Arizti, F., Gomez, E., Aguinaga, A., Del Pozo, J.L., Arana, S. "Smart central venous port for early detection of bacterial biofilm related infections" <b>(2014)</b> <i>Biomed Microdevices</i>, 16: 365.]</ref> Of the above microbes, <i>S. epidermidis</i> is the most relevant port associated pathogen. Jukes et al. estimate in the United States, 80% of hospital acquired catheter related bloodstream infections (CRBSI) are caused by the microbe <i>S. epidermidis</i>.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/20813851/ Jukes, L., Mikhail, J., Bome-Mannathoko, N., Hadfield, S.J., Harris, L.G., El-Bouri, K., Davies, A.P., Mack, D. "Rapid differentiation of <i>Staphylococcus aureus</i>,<i>Staphylococcus epidermidis</i> and other coagulase-negative staphylococci and meticillin susceptibility testing directly from growth-positive blood cultures by multiplex real-time PCR" <b>(2010)</b> </i>J. Med. Microbiol</i>, 59:1456–1461]</ref> The treatment of these catheter-related bloodstream <i>S. epidermidis</i> infections costs the U.S. about $2 billion a year.<ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2807625/ Otto, Michael. “<i>Staphylococcus Epidermidis</i> – the ‘accidental’ Pathogen.” <b>(2009)</b> <i>Nature reviews. Microbiology</i> 7.8: 555–567. <i>PMC<b>.]</ref> Thus, research on practices that decrease chest port infection incidence and basic scientific research on the mechanism of chest infections can significantly reduce the problem of chest port infections. | ||
<i>S. epidermidis</i> is an opportunistic pathogen. <i>S. epidermidis</i> are normally non-pathogenic and found on the human skin.<ref>[https://www.ncbi.nlm.nih.gov/books/NBK7008/ Buttner, H., Dietrich, M. and H. Rohde. "Structural Basis of <i>Staphylococcus Epidermidis</i> Biofilm Formation: Mechanisms and Molecular Interactions." <b>(2015)</b> <i>Frontiers in Cellular and Infection Microbiology</i> 5: 14. <i>PMC</i>.]</ref> S. epidermidis only act as a human pathogen in individuals with compromised immune systems, immunosuppression, or chemotherapy related neutropenia.<ref>[https://www.ncbi.nlm.nih.gov/books/NBK7008/ Buttner, H., Dietrich, M. and H. Rohde. "Structural Basis of <i>Staphylococcus Epidermidis</i> Biofilm Formation: Mechanisms and Molecular Interactions." <b>(2015)</b> <i>Frontiers in Cellular and Infection Microbiology</i> 5: 14. <i>PMC</i>.]</ref> Common port infections include S. epidermidis biofilm formation inside the catheter lumen.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/24515846/ Paredes, J.,Alonso-Acre, M., Schmidt, C., Valderas, D., Sedano, B., Legarda, J., Arizti, F., Gomez, E., Aguinaga, A., Del Pozo, J.L., Arana, S. "Smart central venous port for early detection of bacterial biofilm related infections" <b>(2014)</b> <i>Biomed Microdevices</i>, 16: 365.]</ref> Biofilms are groups of cells that form on various surfaces that produce extracellular polymeric substances (EPS) which protects the microorganisms from antibiotics.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/12194761/ Donlan, Rodney M. “Biofilms: Microbial Life on Surfaces.” <i>Emerging Infectious Diseases</i> 8.9 <b>(2002)</b>: 881–890. <i>PMC</i>. Web. 24 Apr. 2017.] </ref> Biofilm formation is threefold. S. epidermidis adhere to the catheter surface to be colonized, a microcolony forms, and S. epidermidis cells detach from a mature biofilm allowing S. epidermidis colonization on additional body sites. Better management of chest port microbial infections can help improve patient quality of life as they undergo treatment. Recent research explores how to best manage port microbial infections avoiding the current treatment of port excision. The literature focuses on both the mechanism of S. epidermidis pathogenicity/biofilm formation in order to develop better antibiotics and also alternate treatments for port infections. | <i>S. epidermidis</i> is an opportunistic pathogen. <i>S. epidermidis</i> are normally non-pathogenic and found on the human skin.<ref>[https://www.ncbi.nlm.nih.gov/books/NBK7008/ Buttner, H., Dietrich, M. and H. Rohde. "Structural Basis of <i>Staphylococcus Epidermidis</i> Biofilm Formation: Mechanisms and Molecular Interactions." <b>(2015)</b> <i>Frontiers in Cellular and Infection Microbiology</i> 5: 14. <i>PMC</i>.]</ref> S. epidermidis only act as a human pathogen in individuals with compromised immune systems, immunosuppression, or chemotherapy related neutropenia.<ref>[https://www.ncbi.nlm.nih.gov/books/NBK7008/ Buttner, H., Dietrich, M. and H. Rohde. "Structural Basis of <i>Staphylococcus Epidermidis</i> Biofilm Formation: Mechanisms and Molecular Interactions." <b>(2015)</b> <i>Frontiers in Cellular and Infection Microbiology</i> 5: 14. <i>PMC</i>.]</ref> Common port infections include S. epidermidis biofilm formation inside the catheter lumen.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/24515846/ Paredes, J.,Alonso-Acre, M., Schmidt, C., Valderas, D., Sedano, B., Legarda, J., Arizti, F., Gomez, E., Aguinaga, A., Del Pozo, J.L., Arana, S. "Smart central venous port for early detection of bacterial biofilm related infections" <b>(2014)</b> <i>Biomed Microdevices</i>, 16: 365.]</ref> Biofilms are groups of cells that form on various surfaces that produce extracellular polymeric substances (EPS) which protects the microorganisms from antibiotics.<ref>[https://www.ncbi.nlm.nih.gov/pubmed/12194761/ Donlan, Rodney M. “Biofilms: Microbial Life on Surfaces.” <i>Emerging Infectious Diseases</i> 8.9 <b>(2002)</b>: 881–890. <i>PMC</i>. Web. 24 Apr. 2017.] </ref> Biofilm formation is threefold. S. epidermidis adhere to the catheter surface to be colonized, a microcolony forms, and S. epidermidis cells detach from a mature biofilm allowing S. epidermidis colonization on additional body sites. Better management of chest port microbial infections can help improve patient quality of life as they undergo treatment. Recent research explores how to best manage port microbial infections avoiding the current treatment of port excision. The literature focuses on both the mechanism of S. epidermidis pathogenicity/biofilm formation in order to develop better antibiotics and also alternate treatments for port infections. | ||
Revision as of 21:19, 28 April 2017
Introduction
By Hannah Lorico Hertz
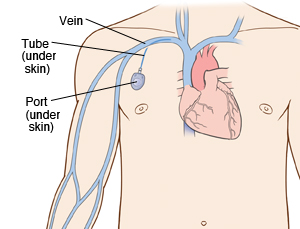
A chest port is an indwelling catheter connected to a reservoir, inserted under the skin of the chest, and used to administer medicines directly into a vein over a long period of time (Figure 1). Physicians frequently utilize chest ports to administer multiple cycles of chemotherapy in children because of the ease of vascular access and care for port maintenance. Because chest ports are inserted under the skin, the skin covering the port acts as a barrier to potentially harmful microorganisms. In comparison to an IV line, chest ports can stay in place for months at a time, can be used to collect blood samples without needles, and have a lower risk of infection over time. Although chest port infections are not as common as other external catheter infections, the most significant complication during chemotherapeutic treatment via chest port catheters are microbial infections. Approximately 5% of patients diagnosed with a microbial port infection are treated by port excision.[1]
Infections of implanted devices most commonly result from Staphylococcus epidermidis, Staphylococcus aureus, Enterococcus faecalis, Streptococcus vidrians, Klebsiella pneumonia, and Pseudomona aeruginosa.[2] Of the above microbes, S. epidermidis is the most relevant port associated pathogen. Jukes et al. estimate in the United States, 80% of hospital acquired catheter related bloodstream infections (CRBSI) are caused by the microbe S. epidermidis.[3] The treatment of these catheter-related bloodstream S. epidermidis infections costs the U.S. about $2 billion a year.[4] Thus, research on practices that decrease chest port infection incidence and basic scientific research on the mechanism of chest infections can significantly reduce the problem of chest port infections.
S. epidermidis is an opportunistic pathogen. S. epidermidis are normally non-pathogenic and found on the human skin.[5] S. epidermidis only act as a human pathogen in individuals with compromised immune systems, immunosuppression, or chemotherapy related neutropenia.[6] Common port infections include S. epidermidis biofilm formation inside the catheter lumen.[7] Biofilms are groups of cells that form on various surfaces that produce extracellular polymeric substances (EPS) which protects the microorganisms from antibiotics.[8] Biofilm formation is threefold. S. epidermidis adhere to the catheter surface to be colonized, a microcolony forms, and S. epidermidis cells detach from a mature biofilm allowing S. epidermidis colonization on additional body sites. Better management of chest port microbial infections can help improve patient quality of life as they undergo treatment. Recent research explores how to best manage port microbial infections avoiding the current treatment of port excision. The literature focuses on both the mechanism of S. epidermidis pathogenicity/biofilm formation in order to develop better antibiotics and also alternate treatments for port infections.
S. epidermidis and Pathogenicity

Biofilm Formation
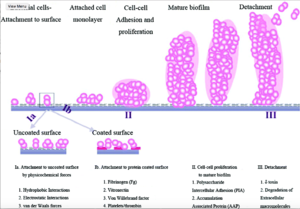
Staphylococcus epidermidis biofilm formation begins with cell attachment to a surface. For port microbial infections, biofilm formation most commonly occurs on the catheter lumen. Next, S. epidermidis begin producing polysaccharides forming a protective layer surrounding the cells. The biofilm continues to grow until the cells are released into the environment, free to begin biofilm formation at another surface. [9]
The primary attachment of S. epidermidis on the surface of the port catheter lumen is essential for the establishment of port-associated infections. Attachment of S. epidermidis can occur either by the direct attachment of the cell to a polymer surface by physio-chemical interactions or by the attachment to extracellular matrix proteins (ECM) coating the surface of a port catheter. Heilmann et al. (1997) [10] identified factors essential for S. epidermidis-polymer surface interactions. The autolysin AtlE is essential for S. epidermidis attachment to polystyrene (plastic).[11] AltE interacts to the surface of the port catheter lumen by hydrophobic interactions, however, further research on AltE mechanism of attachment is needed to eliminate the possibility that AltE plays an indirect role in attachment.[12] In addition, the amino acid sequence of AltE is 61% identical to the AltL gene of Staphylococcus aureus. This similarity enabled Heilmann et al. (1997) to identify the two domains of interest for AltE mediation of attachment to plastic surfaces: a 60kDa amidase and a 52kDa glucosaminidase domain.[13] To test if the 60 kDa AltE domain plays a role in S. epidermidis polystyrene attachment, Heilmann et al. (1997) incubated wild type S. epidermidis with an anti-60 kDa antiserum. If the 60 kDa amidase mediates primary attachment to polystyrene, then the researchers expected to see an inhibitory effect on attachment in the initial adherence assay. As expected, with the incubation of anti-60kDa antiserum, the number of wild-type cells was approximately 90% reduced.[14] This suggests that the 60 kDa domain of AltE plays an important role in S. epidermidis biofilm attachment. AltE plays a significant role in biofilm attachment; Not only does AltE play a role in binding to the polystyrene surface, but also to ECM coated surfaces (specifically vitronectin).[15]
Moreover, as foreign materials are inserted into the body, they become covered by host ECM components and so, as chest ports are inserted in the body, the ports are coated by EMC components such as fibrinogen, vitronectin, collagen, and fibronectin. Research has shown that proteins with ECM-binding activity expressed by S. epidermidis can play a significant role in microbial biofilm initiation of a port infection. Proteins such as AltE bind to vitronectin, lipase GehD to collagen [16] , and surface component serine-asparatate repeat (Sdr) [17] proteins SdrF, SdrG, and SdrH to [18] to collagen I [19], fibrogen, and fibrinogen respectively.
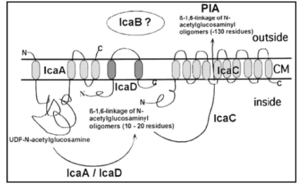
Biofilm accumulation of S. epidermidis begins with the expression of intercellular adhesive properties. The protein, polysaccharide intercellular adhesin (PIA), is the main adhesive expressed by S. epidermidis [20] leading to cell aggregation and biofilm formation. [21] PIA is produced by the membrane proteins IcaA, IcaD, and IcaC, which are expressed by the ica locus.[22] PIA formation begins as IcaA and IcaD form an N-acetylglucosamine transferase. This transferase uses UDP-glucosamine (UDP-GlcNAc) to build unbranched glucosamine (GlcNAc) homopolymers with a length of about 15-20 residues. IcaC produces longer chains forming PIA and is also involved in PIA export. PIA can undergo editing by IcaB, which de-acetylates some of the GlcNac residues, conferring a positive charge to a formerly neutral GlcNAC when in an environment with a pH <6.[23] PIA can then function as an adhesive for S. epidermidis biofilm production.
In addition to PIA, proteins Aap and Embp can also lead to biofilm assembly. Other biofilm associated proteins involved with biofilm accumulation include SesC. [24]
The understanding of S. epidermidis pathogenicity is linked to biofilm formation. Thus, many studies have been conducted studying biofilm formation in vitro. Further research of biofilm systems in complex models are necessary to more accurately portray the in vivo infection of a chest port.
Symptoms and Diagnosis of Infection
Chest port infections most commonly occur in patients with compromised immune systems, immunosuppression, and chemotherapy related neutropenia. The onset of a port infection can be recognized by numerous symptoms. Symptoms include a high fever (≥ 38.3°C or 101°F), redness, tenderness, and induration at port site. With neutropenic patients, sometimes the only detectable symptom of a port infection is fever and chills because of a lack of inflammatory responses.( Sanjeet Dadwal,2015)
The diagnostic workup of an infection includes at least two blood cultures, one from a peripheral vein and one from the port catheter, stool examination for C. difficile and other bacterial/protozoal microbes (if diarrhea is present),urine culture and urinalysis, a chest radiograph, respiratory samples for culture, and the aspiration or biopsy of any skin lesions.
Current Treatments
Better Treatment Methods
Caring for a Port
Conclusion
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduce the topic of your paper. What is your research question? What experiments have addressed your question? Applications for medicine and/or environment?
Include some current research, with at least one figure showing data.
Include some current research, with at least one figure showing data.
References
- ↑ [Funaki, Brian. “Subcutaneous Chest Port Infection.” (2005). Seminars in Interventional Radiology, 22.3: 245–247. PMC.]
- ↑ Paredes, J.,Alonso-Acre, M., Schmidt, C., Valderas, D., Sedano, B., Legarda, J., Arizti, F., Gomez, E., Aguinaga, A., Del Pozo, J.L., Arana, S. "Smart central venous port for early detection of bacterial biofilm related infections" (2014) Biomed Microdevices, 16: 365.
- ↑ Jukes, L., Mikhail, J., Bome-Mannathoko, N., Hadfield, S.J., Harris, L.G., El-Bouri, K., Davies, A.P., Mack, D. "Rapid differentiation of Staphylococcus aureus,Staphylococcus epidermidis and other coagulase-negative staphylococci and meticillin susceptibility testing directly from growth-positive blood cultures by multiplex real-time PCR" (2010) J. Med. Microbiol, 59:1456–1461
- ↑ Otto, Michael. “Staphylococcus Epidermidis – the ‘accidental’ Pathogen.” (2009) Nature reviews. Microbiology 7.8: 555–567. PMC.
- ↑ Buttner, H., Dietrich, M. and H. Rohde. "Structural Basis of Staphylococcus Epidermidis Biofilm Formation: Mechanisms and Molecular Interactions." (2015) Frontiers in Cellular and Infection Microbiology 5: 14. PMC.
- ↑ Buttner, H., Dietrich, M. and H. Rohde. "Structural Basis of Staphylococcus Epidermidis Biofilm Formation: Mechanisms and Molecular Interactions." (2015) Frontiers in Cellular and Infection Microbiology 5: 14. PMC.
- ↑ Paredes, J.,Alonso-Acre, M., Schmidt, C., Valderas, D., Sedano, B., Legarda, J., Arizti, F., Gomez, E., Aguinaga, A., Del Pozo, J.L., Arana, S. "Smart central venous port for early detection of bacterial biofilm related infections" (2014) Biomed Microdevices, 16: 365.
- ↑ Donlan, Rodney M. “Biofilms: Microbial Life on Surfaces.” Emerging Infectious Diseases 8.9 (2002): 881–890. PMC. Web. 24 Apr. 2017.
- ↑ 2007
- ↑ Heilmann C., Hussain M., Peters G., Götz F. "Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface." Mol. Microbiol. (1997) 24, 1013–1024.
- ↑ Bowden M. G., Visai L., Longshaw C. M., Holland K. T., Speziale P., Hook M. "Is the GehD lipase from Staphylococcus epidermidis a collagen binding adhesin?" (2002). J. Biol. Chem. 277: 43017–43023.
- ↑ Heilmann C., Hussain M., Peters G., Götz F. "Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface." Mol. Microbiol. (1997) 24, 1013–1024.
- ↑ Heilmann C., Hussain M., Peters G., Götz F. "Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface." Mol. Microbiol. (1997) 24, 1013–1024.
- ↑ Heilmann C., Hussain M., Peters G., Götz F. "Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface." Mol. Microbiol. (1997) 24, 1013–1024.
- ↑ Heilmann C., Hussain M., Peters G., Götz F. "Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface." Mol. Microbiol. (1997) 24, 1013–1024.
- ↑ Bowden M. G., Visai L., Longshaw C. M., Holland K. T., Speziale P., Hook M. "Is the GehD lipase from Staphylococcus epidermidis a collagen binding adhesin?" (2002). J. Biol. Chem. 277: 43017–43023.
- ↑ McCrea K. W., Hartford O., Davis S., Eidhin D. N., Lina G., Speziale P., et al. "The serine-aspartate repeat (Sdr) protein family in Staphylococcus epidermidis." . (2000) Microbiology 146: 1535–1546.
- ↑ E., McCrea K. W., Ni E. D., O'Connell D., Cox J., Hook M., Foster, T.J. "Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus." (1998). Microbiology 144:3387–3395.
- ↑ Arrecubieta C., Asai T., Bayern M., Loughman A., Fitzgerald J. R., Shelton C. E., Baron, H.M., Dang, N.C., Deng, M.C., Naka,Y., Foster, T.J., Lowy, F.D. "The role of Staphylococcus aureus adhesins in the pathogenesis of ventricular assist device-related infections." (2006) J. Infect. Dis. 193:1109–1119.
- ↑ [Mack D., Siemssen N., Laufs R. "Identification of a cell cluster associated antigen specific for plastic-adherent Staphylococcus epidermidis which is functional related to intercellular adhesion. (1994b) Zentralbl. Bakteriol. Suppl. 26: 411–413.]
- ↑ Costerton J. W., Lewandowski Z., Caldwell D. E., Korber D. R., Lappin-Scott H. M. "Microbial biofilms." (1995). Annu. Rev. Microbiol. 49: 711–745.
- ↑ Heilmann C., Hussain M., Peters G., Götz F. "Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface." Mol. Microbiol. (1997) 24, 1013–1024.
- ↑ Heilmann C., Hussain M., Peters G., Götz F. "Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface." Mol. Microbiol. (1997) 24, 1013–1024.
- ↑ Shahrooei M., Hira V., Stijlemans B., Merckx R., Hermans P. W., Van Eldere J. " Inhibition of Staphylococcus epidermidis biofilm formation by rabbit polyclonal antibodies against the SesC protein." (2009) Infect. Immun. 77: 3670–3678.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
