The Monoxenous Life Cycle Of Eimeria
Introduction
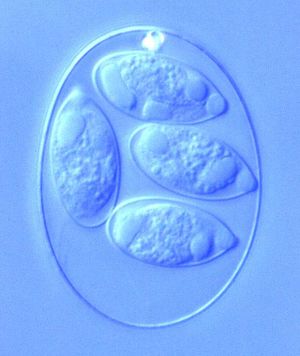
By Emma Stewart-Bates
Eimeria is a genus of protozoa that are parasitic to many vertebrate animals, most often cattle, domesticated birds, goats, and sheep. These parasites contain an apical complexes and apicoplasts, organelles that allow the cell to enter a host organism. The life cycle of Eimeria is considered monoxenous, meaning that the cycle occurs in one host. The three stages of its life cycle include oocyst, sporozoite, and merozoite. They undergo both sexual and asexual reproduction during different stages of their life. Animals infected by Eimeria often develop the disease coccidiosis, which mainly causes diarrhea, fatigue, and loss of appetite. Coccidiosis is spread when an animal ingests infected tissue or is exposed to contaminated feces.[1] The spread of coccidiosis costs the poultry market an enormous amount of money each year. As a result, much research has been conducted on how to manage and treat the outbreak of Eimeria infections. This research includes the benefits and disadvantages of anticoccidial medications, vaccinations, and other treatment measures, as well as how those measures work within the body of the host organism.[2]
Life Cycle
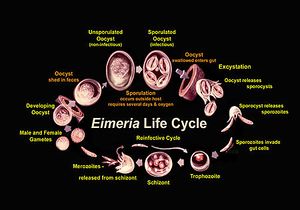
Eimeria are in the oocyst stage when in the environment and during ingestion. The oocyst is in early, unsporulated form when the previous host releases it into the surroundings.[3] The tough outer walls of the oocyst protect it from harmful conditions in the environment.[4] The oocyst enters late phase when it forms spores through asexual production. Four sporocysts (with two sporozoites each) are formed in each oocyst. This spore formation requires an aerobic environment and takes about one day. These oocysts are then consumed by an animal and reach the intestine, where they are broken down with the help of stomach matter such as bile, trypsin, and CO2. The newly-formed sporozoites are circulated in the intestine, where they invade epithelial cells in the walls. Sporozoite development can occur in those cells or at another location (intestinal crypts) depending on the species.[5] The sporozoites are gathered as a trophozoite and become larger to become a schizont.[6] The schizont expels merozoites, which leave the cell to invade other epithelial cells. This cycle continues for a few more generations, after which the merozoites transform into male or female forms and perform sexual reproduction. The result is an early, unsporulated oocyst, which is released by the animal in the form of feces.[7] Thus, the cycle begins again. The entire cycle lasts about 4-7 days and varies among species.[8]
Phase Morphology
The different phases within the life cycle of Eimeria have different morphologies to serve the functions of the organism. Eimeria oocysts are shaped like an oval or elliptical and are typically 10-40 μm. The oocyst is protected from potential dangers in the surroundings by a tough cell wall. Thus, wall-forming structures are contained in the cell, as well as polar caps, micropyles, and other bodies. Oocysts also have sporoblasts when unsporulated, which are turned into sporocysts (4 per cell, each containing 2 sporozoites) after sporulation. Once inside a cell in the host organism, the schizont forms to appear as a round cell full of bodies. The size of the schizont relies on the species of Eimeria, the place in the host organism, and the amount of development completed, but they are usually between 10-100 μm. Occasionally they become very large, reaching to about 1 mm. Finally, the gamonts have two forms, microgamont (male) and macrogamont (female). The microgamonts have multiple nuclei and disperse gametes with two flagella, while the macrogamont contain one oval-shaped nucleus.[9] The size, shape, and structure of each of these bodies serves the main function of the protozoa during that particular stage of life.
Metabolism
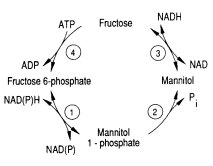
One of the pathways for metabolism found in Eimeria is the mannitol cycle, which mainly occurs during the sexual part of their life cycle. However, it is present in different phases of the cycle as well. The mannitol cycle originates as a branch from glycolysis at fructose-6-phosphate,[12] and was originally thought to only occur in fungi. There are four enzymes responsible for the conversion between fructose 6-phosphate and mannitol, including mannitol-1-phosphate dehydrogenase, mannitol-1-phosphatase, mannitol dehydrogenase, and hexokinase. The most important of these enzymes is likely mannitol-1-phosphate dehydrogenase because it is the connection to glycolysis, the pentose phosphate pathway, and the mannitol cycle.[13]
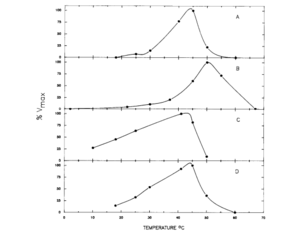
This cycle was explored in 1988 by D.M. Schamntz et al. in E. tenella. Their findings indicate that this cycle occurs in one direction due to the buildup of fructose and shortage of mannitol kinase. Mannitol is likely formed as the oocysts are being produced and builds up to provide energy for oocyst sporulation outside of the host organism. The idea that mannitol is formed as the oocysts are formed was supported by the fact that the enzymes for the mannitol cycle were much more active at the temperature level of the body of the host organism (chickens, in this case) than at any other temperature, as shown in the figure. In addition to providing energy for oocyst sporulation, mannitol may give energy for the oogenesis pathway so that it can bear the oxygen-poor gut environment. The first half of the cycle can serve as an electron sink when NADPH is oxidized when fructose-6-phosphate is reduced to mannitol-1-phosphate.[14] Thus, the mannitol cycle, which cycles four different molecules with four different enzymes, is essential to powering the survival and sporulation of Eimeria at different phases in their life cycle.
Infection and Diagnosis
Coccidiosis, the disease that can result from Eimeria oocyst ingestion, affects a wide range of animals including mammals, birds, reptiles, and fish. The infection begins when the animal ingests a sporulated Eimeria oocyst and is spread as animals excrete new oocysts in their feces. Typically, each species of Eimeria infect a single species of host, but many different species may infect that same type of host. For example, there are over 10 Eimeria species that infect cattle and over 10 that infect sheep. Other popular host species include chickens, turkeys, goats, pigs, rabbits, and dogs. Some Eimeria species may infect hosts that are closely related to their preferred host species. The normal infection point is the intestine, but can also include the liver, gallbladder, and kidneys depending on the species.[15] Compared to the number of animals infected, the number of animals that actually show symptoms is relatively low. However, the signs of infection can include diarrhea, fever, weight loss, malnutrition, and death. Excretion of blood or tissue may also be seen. The animals that are most susceptible to coccidiosis are young animals contained in a small space with poor sanitation.[16] The severity of the disease depends on certain factors such as the species that is infecting (some are more harmful than others) and host characteristics (susceptibility due to genetic factors). The chances of infection are increased with forms of stress like unsatisfactory food, insufficient space, poor hygiene, and other infectious agents like bacteria.[17]
The veterinarian method of sugar or salt flotation can be utilized to determine whether feces is contaminated with Eimeria oocysts, which requires the observer to understand what the oocysts look like.[18] This method involves using sugar and salt solutions to separate parasitic eggs from the fecal matter. The sugar and salt are necessary because the eggs sink in water, but float in these solutions.[19] This method is not always successful in determining infection because oocysts may not be expelled by the animal until days after infection. Lesion scoring is an additional way to determine the presence of Eimeria in an animal; this process involves examination of noticeable macrolesions on the gut caused by Eimeria. As with the flotation method, lesion scoring requires interpretation by a trained professional and is still subjective. Also, the presence and meaning of microlesions makes the process of lesion scoring more complex.[20] Another important aspect that must be considered is whether the species is pathogenic to that specific host.[21] As a result, determining whether or not an animal is infected with Eimeria can be a complicated and inconsistent process.
Recent technological advances could also help in the diagnosis of coccidiosis. For example, certain electrophoresis procedures, sourthern blot assays, and PCR techniques can be used to diagnose the disease and determine Eimerian genetic variation, although the expense of these procedures limits the prevalence of their use.[22] However, an online database COCCIMORPH analyzes different species of coccidia based on morphological traits like size, curvature, symmetry, etc., which can be very useful in diagnosis.[23]
Effects on the Body
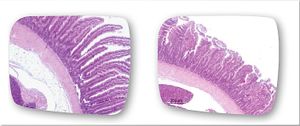
While many of the signs of coccidiosis are very distinguishable (such as diarrhea, weight loss, dehydration, etc.), the origin of these signs stems from the way in which Eimeria interact with the host cells and the way they harm them. A study by Frietas (2014) examined this interaction by infecting chickens with Eimeria maxima through experimental injections. The Eimeria maxima caused injury to the digestive tract of the animal, resulting in a decreased ability for absorption in the intestines. In particular, the pathogens damaged the villi in the gut, hindering the absorption of many important nutrients like glucose, zinc, calcium, phosphorus, as well as other proteins and lipids. Typically, Eimeria are able to damage the function of the gut by shortening the length of the villi and thus decreasing the villi-to-crypt ratio within the intestines.[25] This can even occur in species that used to be considered non-pathogenic, like E. praecox (as shown in the figure).[26] Another effect of Eimeria infection is a decreased pH level in the intestine, leading to slower activity of the hydrolases. Infection also influences the jejunum mucosa, resulting in alterations in lipid absorption. This destruction of the intestinal villi accounts for the diarrhea and weight loss as well as the disruption of the necessary flow of nutrients and water within the body. A further complication of this damage is that it can destroy an innate defense of the body and create an opportunity for more pathogens to thrive.[27] Examination of the bodies of animals that did not survive Eimeria infection show thin, semi-clear gut walls, swollen gut, decreased rigidity of the intestinal muscles, watery and undigested material in the gut, and an oily, colorful appearance of the contents near the mucosa.[28] The interactions that Eimeria cells have with the host cells can lead to huge damage to the gastrointestinal system of the host, resulting in painful and occasionally deadly symptoms.
Treatment and Prevention
Impact on Poultry Market
Immunization
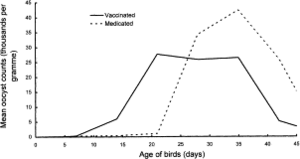
Conclusion
References
- ↑ "Eimeria." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.
- ↑ Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." Clinical Microbiology Reviews 15.1 (2002): 58-65. Web.
- ↑ Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." Clinical Microbiology Reviews 15.1 (2002): 58-65. Web.
- ↑ "Eimeria." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.
- ↑ Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." Clinical Microbiology Reviews 15.1 (2002): 58-65. Web.
- ↑ Johnstone, Colin. “Eimeria bovis.” University of Pennsylvania. 24 January 2000. Web. 15 April 2017.
- ↑ Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." Clinical Microbiology Reviews 15.1 (2002): 58-65. Web.
- ↑ “Eimeria biological cycle: an example of perfect complexity in biology.” Eimeria Prevention. HIPRA, 30 May 2016. Web. 19 Apr. 2017.
- ↑ "Eimeria." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.
- ↑ Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in Eimeria tenella.” Molecular and Biochemical Parasitology 32.2-3 (1989): 263-270.
- ↑ Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in Eimeria tenella.” Molecular and Biochemical Parasitology 32.2-3 (1989): 263-270.
- ↑ Schmatz, D.M. “The mannitol cycle in Eimeria.” Parasitology (2002): 114. Web.
- ↑ Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in Eimeria tenella.” Molecular and Biochemical Parasitology 32.2-3 (1989): 263-270.
- ↑ Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in Eimeria tenella.” Molecular and Biochemical Parasitology 32.2-3 (1989): 263-270.
- ↑ "Eimeria." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.
- ↑ Constable, Peter D. "Overview of Coccidiosis." Veterinary Manual. Merck Sharp & Dohme Corp., 2016. Web. 15 Apr. 2017.
- ↑ Ahmad, Tarek A., Bassant A. El-Sayed, and Laila H. El-Sayed. “Development of Immunization Trials against Eimeria spp.” Trials in Vaccinology 5(2002): 38-47. Web.
- ↑ Constable, Peter D. "Overview of Coccidiosis." Veterinary Manual. Merck Sharp & Dohme Corp., 2016. Web. 15 Apr. 2017.
- ↑ Corwin, RM, and Julie Nahm. “Veterinary Parasites Laboratory Procedures.” University of Missouri College of Veterinary Medicine. 1997. Web. April 15, 2017.
- ↑ De Gussem, M. “Coccidiosis in poultry: review on diagnosis, control, prevention and interaction with overall gut health.” Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, 26-30 August, 2007, pp. 253-261.
- ↑ Constable, Peter D. "Overview of Coccidiosis." Veterinary Manual. Merck Sharp & Dohme Corp., 2016. Web. 15 Apr. 2017.
- ↑ De Gussem, M. “Coccidiosis in poultry: review on diagnosis, control, prevention and interaction with overall gut health.” Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, 26-30 August, 2007, pp. 253-261.
- ↑ Gruber, A., and Pereira, C.A.B., Costa, L.F., and Castañón, C.A.B.. COCCIMORPH - Coccidiosis Diagnosis through Morphological Analysis. N.p., 2007. Web. 24 Apr. 2017.
- ↑ https://eimeriaprevention.com/news/coccidiosis-in-chickens-and-subclinical-species-of-eimeria/ “Coccidiosis in chickens: the role of subclinical species of Eimeria.” Eimeria Prevention. HIPRA, 16 Sept. 2016. Web. 19 Apr. 2017.]
- ↑ Frietas, F.L.D.C. “Metabolic alterations in broiler chickens experimentally infected with sporulated oocysts of Eimeria maxima.” Veterinary Parasitology Laboratory 23.3 (2014): 309-314. Web.
- ↑ “Coccidiosis in chickens: the role of subclinical species of Eimeria.” Eimeria Prevention. HIPRA, 16 Sept. 2016. Web. 19 Apr. 2017.
- ↑ Frietas, F.L.D.C. “Metabolic alterations in broiler chickens experimentally infected with sporulated oocysts of Eimeria maxima.” Veterinary Parasitology Laboratory 23.3 (2014): 309-314. Web.
- ↑ De Gussem, M. “Coccidiosis in poultry: review on diagnosis, control, prevention and interaction with overall gut health.” Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, 26-30 August, 2007, pp. 253-261.
- ↑ http://www.sciencedirect.com/science/article/pii/S0020751998002124 Williams, R.B., W.W.H. Carlyle, D.R. Bond, and I.A.G. Brown. “The efficacy and economic benefits of ParacoxⓇ, a live attenuated anticoccidial vaccine, in commercial trials with standard broiler chickens in the United Kingdom.” International Journal for Parasitology 29(1999): 341-355. Web..]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
