Human Papilloma Virus


Etiology/Bacteriology
Taxonomy
|Family = Papillomaviridae
|Genus = Alphapapillomavirus
|species = Human Papillomavirus
NCBI: Taxonomy Genome: Genome
Description
Papillomavirus are small virus with DNA from the family papovavirdae which measure 50 nm in diameter, lack membrane, and their capsids have an icosahedral form composed of 72 capsomeres [3].The DNA of HPV is circular, double chain, and contains approximately 8000 base pairs [3]. Persistent infection by human papilloma virus (HPV) is considered the main causative agent of cervical cancer. According to their oncogenic risk, HPV are classified as low-risk HPV (LR-HPV) or high-risk HPV (HR-HPV) [3]. Classification is based on the origin and degree of homogeneity of the DNA.
Pathogenesis
Transmission
Transmission of HPV occurs most commonly during sexual activity. During sexual activity, micro-trauma or the genital epithelium, particularly in the transformation areas of the cervical epithelium, allows exposure of basal cells to active proliferation of the various types of HPV [3]. The union between receptor of the basal cells with the protein of the HPV infection occurs via direct contact between skin and mucous membranes. Though sexual relationships are the main form of contact, transmission can occur between mother and child during the time of childbirth [3]. In order for infection to develop, the complete virus must be transmitted and not only DNA fragments.
Steps of Pathogenesis
HPV only infects dried epithelium and mucous membranes. Once in its niche, HPV is often cleared by the immune system and no further complications arise. If not cleared by the immune system the infection will induce the formation of benign lesions (warts or papillomas) and under certain conditions and in partnership with certain cofactors carcinoma will be produced [3]. In order for HPV to penetrate and initiate the infectious process the virus must come in contact with the permissive cells (basal cells of the epithelium). Often, this exposure occurs via the micro-tears formed during sexual activity. Once reaching the permissive cells, viral replication in the spinous layer begins. Expression of early viral genes occurs through the non-encoding region, which allows DNA to produce hundreds of copies per cell [3]. The assembly of the virions is carried out in the upper strata of the epithelium when the cells have been differentiated into granular cells. This stage in the viral cycle is known as the proliferative or productive phase. It is possible that during this stage of amplification of the viral genome, the inhibition of oncoproteins E6 and E7, damage to the cytoskeleton, mitochondrial energy disorder, and apoptosis allow for greater spread of the viral progeny, provided that the proteins in the viral capsid (L1 and L2) have been synthesized, forming new infectious virions with the viral DNA [3]. Apoptosis of the squamous cells causes virions to be expelled. HPV may start a new infection cycle by joining to the cell surface and embedding into the host cytoplasm via endocytosis. After which, the viral cycle begins again.
Virulence Factors
The virulence factors of HPV include proteins E6 and E7 that are heavily present in high risk papillomavirus. These proteins have been shown to inactivate the host's tumor suppressor proteins p53 and Rb. This inactivation of tumor suppresser proteins results in the host cells dividing at rate that cannot be regulated. In HR-HPV malignant transformation occurs in the quickly proliferating cells and causing the formation of cervical cancer [4]
Epidemiology
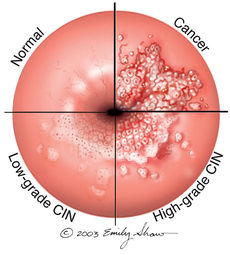
In 1976, Harald zur Hausen was the first to report and study HPV and its participation in carcinogenesis [3]. It is estimated that, in sexually active populations, HPV is ubiquitous. However, 80 per cent of infections are transitory and removed from the immune system without leaving injury. The other 20 percent may progress to form precursors of genital cancers [3]. The prevalence of HPV infections in the general population are very diverse. The complication of the infection is often determined by the type of HPV contracted. HPV constitutes the most frequent sexually transmitted infection in the world. Epidemiological studies have shown that more than one half of male partners of women with cervical intraepithelial neoplasia (CIN) caused by an HPV infection presented penile lesions. If left untreated, precancerous lesions can evolve into invasive carcinoma. In males, squamous cell carcinoma or carcinoma of the penis constitutes more than 1 percent of all the malignancies in men in developed countries, and from 10 to 20 percent in underdeveloped with an incidence of 0.3-1 per 100,000 men [3]. HPV complications in men are rare for those circumcised during the neonatal period.
Clinical Features
Symptoms
There are over 100 kinds of HPV and not all types cause health problems. Some kinds of HPV cause cervical cancer which often does not present with any obvious physical symptoms. Other kinds of HPV can cause genital warts, which favor moist genital surfaces such as the entrance of the vagina and rectum in women. Genital warts can appear to be "small, flat, flesh-colored bumps or tiny, cauliflower-like bumps" [4]. In some cases, genital warts may be too small to see and will need to be further examined. The symptoms of genital warts can include itching, tenderness, burning, pain, or may not even present symptoms at all [4]. Genital warts may appear within weeks or months after having sexual contact with an infected person, or may not appear at all. Genital warts are most commonly found on the vulva, in or around the anus or vagina, on the cervix, the penis, thigh, groin, or scrotum [4].
Diagnosis
The detection of HPV DNA through Molecular Biology techniques, regardless of the method used, is based on the specificity of the complementarity between the nucleic acids [3]. Since HPV infects both men and women, how doctors identify HPV infections vary depending on sex. In women, yearly recommended Pap tests are administered which collect cells from the cervix to be tested for signs of abnormal change [4]. An HPV infection is based on the detection of capsid protein or of specific viral nucleic acid [4]. The Pap test can detect common "high" and "low-risk" HPV genotypes. This distinction allows a physician to appropriately respond to the severity of the infection. There is currently no FDA-approved test to diagnose HPV or the related cancers it may cause in men however, HPV-related cancers are especially rare in men [4].
Treatment
Once an individual contracts HPV, there is no treatment for the actual virus, but those who have a good immune system will usually fight off HPV on their own [4]. Genital warts associated with low-risk HPV types have many different treatment options depending on the location and size of the warts [4]. If the warts are small and managable, a doctor may prescribe a medication in the form of a cream to apply to the warts. Other methods to treat genital warts include cryotherapy (freezing the warts with liquid nitrogen), laser treatment, alpha-interferon injections, or surgical removal [4]. Removal of the warts may not be 100% effective because the viral infection may still be present in the underlying tissue [4]. In cases of high-risk HPV types, most commonly found in women, there are also various treatment plans if there are abnormal changes in the cells of the tested area. Sometimes the best treatment is to just observe and wait to see if the abnormal, or "precancerous cell changes," will heal on their own. Other treatment plans include cryotherapy (freezing the abnormal cells with liquid nitrogen), conization (removal of abnormal areas), or LEEP (Loop Electrosurgical Excision Procedure) to remove the abnormal cells with an electrical current. [4].
Prevention
Associated cofactors related to increased HPV infection include multiple sexual partners, the presence of other STDs, poor hygiene, poor nutrition, smoking, and other conditions that may suppress the immune system [4]. Immunosuppressed patients are at high risk of viral progression and relapse than healthy patients [3]. To prevent contracting HPV, the most effective way is to avoid having sex with an infected person. If your sexual partner is infected with HPV, condoms may reduce the risk of contracting HPV but condoms are not always effective because they may not cover the entirety of the infected area [4]. A successfully resolved prior infection with HPV appears to protect against further infection with that HPV type [5]. Vaccines recently developed to prevent HPV16 and HPV18 associated anogenital cancer consequent of persisting HPV infection induce humoral immune responses to conformational epitopes on the HPV16 and HPV18 viral capsid that are sufficient to provide long lasting protection against viral challenge [5].
Vaccination
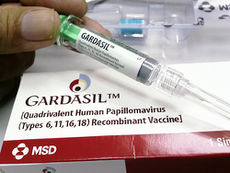
There are three HPV vaccines available to the public as certified by the FDA (Cervarix, Gardasil, and Gardasil 9). Two of the HPV vaccines (Gardasil and Gardasil 9) also protect against genital warts and anal cancers in both males and females. Women should receive the vaccination to prevent cervical cancer, vulvar cancer, vaginal cancer, and genital warts [6]. Males should get the vaccination to prevent anal cancer and genital warts [6]. HPV vaccines are given as a series of three shots over 6 months to protect against the HPV infection and the health risks associated with the infection. The HPV vaccinations offer the best protection to girls and boys who receive all three vaccination doses and have time to develop an immune response before being sexually active [6]. The HPV vaccination is recommended for preteen girls and boys between ages of 11 and 12 years. Young women may receive the vaccine through age 26, and young men through age 21 [6]. The vaccine is also recommended for any mean who has had sex with other men through age 26 [6].
Host Immune Response
Human papillomavirus (HPV) infections are generally long lasting, and a host immune response to infection is hard to detect[5]. Nevertheless immunocompromised subjects control HPV infection less well than those with intact immunity[5. Immune responses are best documented for the papillomavirus groups that cause evident human disease, particularly those responsible for cervical cancers and genital warts[5. Humoral immunity to the viral capsid has been shown sufficient for protection against infection, while innate and adaptive cell mediated immunity appears important for eventual elimination of HPV infection[5]. Infection with a pathogenic papillomavirus produces a humoral immune response to the virus capsid which recognises predominantly conformational determinants displayed only when the L1 protein is correctly configured into pentamers as in the native virus [5].. The adaptive immune response to HPV is slower to appear than that to most pathogenic virus infections [5].
Papillomavirus infections are slow to induce measurable immune responses and slow to clear, suggesting that papillomavirus may have developed methods to evade host immune mechanisms[5]. The primary mechanism of viral immune evasion for PV infection is likely avoidance of antigen presentation — absence of cell lysis and systemic viremia minimises antigen availability for presentation, and also ensures that few pro-inflammatory signals are given in the course of infection to invoke adaptive immune responses. Expression of the capsid proteins is limited to superficial epithelial cells, a consequence of codon usage optimised for expression in differentiated cells and this is likely to reduce presentation of capsid proteins to the immune system [5]. Papillomavirus proteins also apparently encode specific functions to inhibit immune responses[5].
The majority of PV infections do not kill their host or impair host reproductive fitness. Lack of damage to host cells ensures a minimal immune response from the host, enabling vegetative infections that last months to years, with consequent opportunity for viral infection to propagate to many new susceptible hosts[5].
Damage Response Framework
It is difficult to predict the precise Damage Response Framework for HPV due to the variety of strains present. Each strain produces a different host response. Many HPV strains are cleared naturally from the host with no need for medical intervention. The median clearance time for nononcogenic strains of HPV is approximately 4.3 months [7]. Oncogenic strains, however, often require extensive medical attention to remove. With proper medical intervention, oncogenic HPV strains are have a median clearance time of 9.8 months [7]. Without medical intervention, oncogenic HPV strains may not clear on their own and cause eventual complication with possible death.
References
1. College of Arts and Sciences | The University of Oklahoma. Study Abroad.
2. Virology.wisc.edu. 2002. Atomic Model of papillomavirus capsid.
3. Alba A, Cararach M, Rodriguez-Cerdeira C. 2009. The Human Papillomavirus (HPV) in Human Pathology: Description, Pathogenesis, Oncogenic Role, Epidemiology and Detection Techniques. TODJ 3:90-102.
4.Galinski C. Human Papillomavirus (HPV). Austin Community College.
5.Frazer I. 2009. Interaction of human papillomaviruses with the host immune system: A well evolved relationship. Virology 384:410-414.
6. Prevention C. 2015. HPV | Who Should Get Vaccine | Human Papillomavirus | CDC. Cdc.gov.
7. Giuliano A, Harris R, Sedjo R, Baldwin S, Roe D, Papenfuss M, Abrahamsen M, Inserra P, Olvera S, Hatch K. 2002. Incidence, Prevalence, and Clearance of Type‐Specific Human Papillomavirus Infections: The Young Women’s Health Study. The Journal of Infectious Diseases 186:462-469.
8. Vacciniinforma.it. 2014. Vaccini Informa.
9. Mobieg.co.za. 2015. Human Papilloma Virus | MobieG.
Created by Hannah Wilson (student of Tyrrell Conway at the University of Oklahoma).