Acholeplasma laidlawii
A Microbial Biorealm page on the genus Acholeplasma laidlawii
A Microbial Biorealm page on the genus Acholeplasma laidlawii
Classification
Higher order taxa
Bacteria; Firmicutes/Tenericutes; Mollicutes; Acholeplasmatales; Acholeplasma
Species
|
NCBI: Taxonomy |
Acholeplasma laidlawii
Description and significance
Morphologically and microbiologically, Mollicutes are classified as Bacteria that were probably derived from lactobacilli, bacilli or streptococci by regressive evolution and genome reduction, to produce the smallest and simplest free-living and self-replicating cells. Their lifestyle is, in general, parasitic. Structurally, Mollicutes are characterized by the complete lack of cell wall and the presence of an internal cytoskeleton (12).
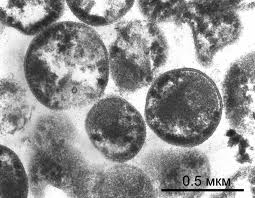
A distinctive trait of A. laidlawii in comparison to other mollicutes is the lack of a cell wall (4). It is also a pigmented with neurosporene -C40, a carotenoid pathway pigment which is synthesized by acetate (4). The cell membrane itself differs from other mollicutes in that the main components are comprised of glycolipids and acholeplasma-specific lipoglycans; sphingosine-1-phosphate is also a component, and cholesterol is not (4). The incredibly small size of this organism (less than 0.2 micrometers) contributes to its ability to contaminate almost any cell culture media, whether it be serum-free, filtered, or otherwise (7). This is leading to several developing methods of improving decontamination and filtration in biopharmaceutical operations (7).
As one of the most adaptive mollicutes, it is no surprise that A. laidlawii was one of the first mycoplasmas to be cultivated on an artificial, agar- based growth plate(4). Originally isolated from wastewaters in 1936 by its namesake, Laidlaw (4), this particular mycoplasma can be found in almost any habitat, free-living or as a parasite. Members of the Acholeplasma genus have been found in almost every type of living organism, including water fowl, crusteaceans, mammals, and reptiles. It receives nutrients from whatever medium it inhabits- whether it be Tryptic Soy Broth in a lab or the gills of the mud crab Scylla serrata (1, 7) It is an important organism to study due to its ability to contaminate almost any sort of medium in a lab, serum-free or not, as well as its ability to avoid detection by routine filtration and test-kit procedures (8). About 15-35% of all cultured bacterial or eukarial cell lines are infected with a limited number of Acholeplasma strains that are either swine, bovine, or human in origin(6). This is a serious problem for biopharmaceutical production companies, as the presence of Acholeplasma laidlawii in cell cultures may distort results, and potentially spread certain viruses that could come into contact with patients(7). Although not considered a pathogenic organism to humans, it may cause opportunistic infections; the only recorded incidence of direct human interaction was from an infected burn wound (7).
Genome structure
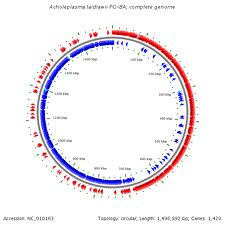
The complete genome of Acholeplasma laidlawii consists of a single, circular chromosome of 1,469,992 base pairs (longest known of the mollicutes) (4). It contains two mRNA operons, 34 tRNA genes, and 1,380 ORFs. It is one of the only mollicutes to use UGA as a stop codon, indicating A. lailawii as a user of the universal code. The G+C content is approximately 31%, and there are no plasmids (4,9). There is no distinctive G-C skew inversion, which usually indicates the oriC region. However, there is an recF gene present which distinguishes the oriC region of A. laidlawii from other mycoplasmas (4). Production of survival bodies called ultramicroforms (UMFs) contain genetic material needed for survival in stress conditions, which only develop when the medium A. laidlawii is in becomes minimal or dried out (7). The genome has 803 verified identified proteins (58%) in its total genome (4,11): 133 genes are involved in translation, 69 involved in transcription, 47 are intergral membrane protein codes, and the rest are hypothetical proteins whose function has yet to be identified (4). The genome of A. laidlawii has regulatory RNA structures, TATA boxes and riboswitches (structures that, upon bindign to ligands, lead to premature termination of transcription or inhibition of translation; regulation mechanisms of DNA replication). There are four kinds of riboswitches present in the genome: flavinmononucleiotide (FMN)- dependent, thyamine pyrophosphate responsive, purine dependent and yybP-ykoY elements (4). There are 19 T boxes upstreams of genes encoding for aminoacyl-tRNA synthases, ABC-type transporters and enzymes, which is common in gram negative bacteria (4).
Cell and colony structure
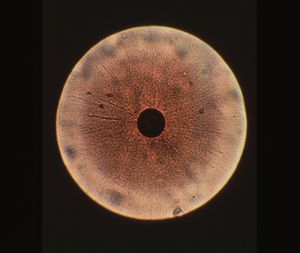
Colony structure has been described as grainy, with typical fried-egg appearane of growth (i.e, dense central button grows down while other cells spread out on surface)(1,8). Cell structure is micrococcal, non-motile, and ranging in size between 0.2 and 0.45 micrometers (9). During starvation or in stressed environments, UMF production increases and colony morphology changes to small, specified clusters ranging between 50 and 300 mircometers in size (2).
Metabolism
Acholeplasma laidlawii has a pronounced metabolic dependence on external media sources, such as culture medium, host cells, etc (i.e., it is a heterotroph). It is a facultative anaerobe (9). Unlike other mycoplasma, they do not require sterols for cultivation, as they are able to synthesize the Fatty Acid Precursor in vitro. Glucose is the only needed carbon donor for carbohydrate synthesis, although fructose and galactose may be used (4). This organism has a complete set of carotenoid pathway proteins, and the only source of ATP it requires is made through the glycolysis pathway, of which it also has the complete set of enzymes (4). Acholeplasma laidlawii is also able to ferment pyruvate into O-lactate, and through transformation of acetyl-CoA, acetic acid, which is required for the carotenoid pathway (4). A. laidlawii can also catabolize NAG and NAM, sugars, and several other amino acids. It is able to break down starch as a carbon source as well, by processing it into glucose-6-phosphate. NAD+ is used for reduction, as indicated by the presence of glutamine-dependent glutamate dehydrogenase and NAD+ synthase. NAD+ is also gathered from nicotamide. Other vitamins provide precursor molecules used for synthesis: 1-carbon pools from folate, coferment A from 4-phosphopantethene and FAD synthesis from flavinmononucleiotides (4). Acholeplasma laidlawii also has several enzymes for complete de novo biosynthesis of aromatic amino acids (F,Y and W), as well as enzymes involved in methionine metabolism (Lysine biosynthesis from Aspartic Acid) (4).
Ecology
Acholeplasma laidlawii is the only mollicute that is capable of existing free of any host (4). However, it requires uptake of nutrients and building blocks for cell processes from the environment- it has most of the required enzymes for metabolising and processing raw materials, but not the precursor elements, deeming it fastidious (4). It is one of the top five contaminating species of cell culture media in laboratories to date (7).
Pathology
A. laidlawii is considered a non-pathogenic microorganism. It causes Clearwater disease in the gills of the Asian Mud Crab Scylla serrata, and it is susceptible to the viruses MV-L1, MV-L2, and MV-L3(1, 5). Almost any animal, vertebrate or invertebrate, is a potential host(1). It creates survival bodies called ultramicroforms that enhance pathogenic factors in the organism due to stressors, such as starvation responses or other infections(5).
References
[1] Chen JG et al. Isolation and Identification of Acholeplasma sp. from the Mud Crab, Scylla serrata. Evid Based Complement Alternat Med. 2011;2011:209406. Epub 2011 Jul 17. PMID:21808652
[2] Chernov, V.M., et al. Unadapted and Adapted to Starvation Acholeplasma laidlawii Cells Induce Different Responses of Oryza sativa, As Determined by Proteome Analysis. Journal of Proteonomics. 18 Novmeber 2011. 74:12, 2920-2936. DOI: 10.1016/j.jprot.2011.07.016
[3] Congdon, Alice L., and Kenny, George E. Alteration of Colonial Morphology of Acholeplasma laidlawii and Acholeplasma modicum by Infection with Mycoplasmatales Viruses. Journal of Bacteriology. 1979. 79, 962-968. PMCID: PMC218128
[4] Lazarev, V.N, et al. Complete Genome and Proteome of Acholeplasma lailawii. Sept. 2011 Journal of Bacteriology. 4943-53. PMID: 21784942
[5] Steinick, L.E., Wieslander, A., Johansson, K.E., and Liss, A. Membrane Composition and Virus Susceptibility of Acholeplasma laidlawii. Journal of Bacteriology. 1980. 80, 1200-1207.
[6] Uphoff, C.C., and Drexler, H.C. Comparative PCR Analysis for Detection of Mycoplasma Infections in Continuous Cell Lines. In Vitro Cell Dev. Biol.- Animal. 2002. 38, 79-85. PMID: 11928999
[7] Windsor, H.M, Windsor G.D., Noordergraaf, J.H. The Growth and Long Term Survival of Acholeplasma laidlawii in Media Products Used in Biopharmaceutical Manufacturing. 2010, Biologicals, 38 (2), 204-210.
[8] Wang, H., Kong, F., Jelfs, P., James, G., and Gilbert, G.L. Simultaneous Detection and Identification of Common Cell Culture Contaminant and Pathogenic Mollicute Strains by Reverse Line Blot Hybridization. Applied and Environmental Microbiology. 2004. 04, 1483-1486. PMCID: PMC368316
[9] Acholeplasma laidlawii PG8-A GOLD Card. University of California Metadata Coverage Index. Updated Sept. 23, 2011.
[10] EMBL-EBI. European Bioinformatics Instituted. 2012.
[11] PATRIC: Genome Overview of Acholeplasma laidlawii PG8. Virginia Bioinformatics Institute.
[12] Wolf, M., Muller, T., Dankedar, T., Pollack, JD. Phylogeny of Firmicutes With Special Reference to Mycoplasma (Mollicutes) As Inferred From Phosphoglycerate Kinase Amino Acid Sequence Data. Int J Syst Evol Microbiol. 2004 May; 54(pt 3): 871-5. PMID: 15143038
Edited by Abigail Felker, student of Dr. Lisa R. Moore, University of Southern Maine, Department of Biological Sciences, http://www.usm.maine.edu/bio