Alcanivorax borkumensis: Difference between revisions
No edit summary |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
=Classification= | =Classification= | ||
| Line 45: | Line 46: | ||
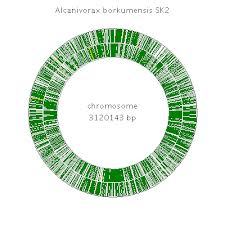
[[File:genome sk2.jpg|300px|thumb|right| Sequenced genome of A. borkumensis SK2 [4F] ]] | [[File:genome sk2.jpg|300px|thumb|right| Sequenced genome of A. borkumensis SK2 [4F] ]] | ||
[[File:alk’s.jpg|300px|thumb|right|Hydroxylation of alkanes by AlkB1 and AlkB2 allows for the degradation of oil by A. borkumensis [5F] ]] | [[File:alk’s.jpg|300px|thumb|right|Hydroxylation of alkanes by AlkB1 and AlkB2 allows for the degradation of oil by A. borkumensis [5F] ]] | ||
As with all vital systems of microorganisms, enzymes are the critical factors which support the function of these three main systems. A. borkumensis produces the enzymes AlkB1 and AlkB2 [[#References|[9]]]. Both are alkane hydroxylases [http://en.wikipedia.org/wiki/Hydroxylation hydroxylation] but AlkB1 oxidizes linear or branched alkanes in the range of five to twelve carbons, whereas AlkB2 oxidizes linear or branched alkanes in the range of eight to sixteen carbons. The importance of AlkB1 and AlkB2 can be appreciated by the fact that both genes encoding the enzymes are located close to oriC [http://en.wikipedia.org/wiki/Origin_of_replication origin of replication], and are thus one of the first genes to be replicated [[#References|[2, 9]]]. | As with all vital systems of microorganisms, enzymes are the critical factors which support the function of these three main systems. A. borkumensis produces the enzymes AlkB1 and AlkB2 [[#References|[9]]]. Both are alkane hydroxylases [http://en.wikipedia.org/wiki/Hydroxylation hydroxylation] but AlkB1 oxidizes linear or branched alkanes in the range of five to twelve carbons, whereas AlkB2 oxidizes linear or branched alkanes in the range of eight to sixteen carbons. The importance of AlkB1 and AlkB2 can be appreciated by the fact that both genes encoding the enzymes are located close to oriC [http://en.wikipedia.org/wiki/Origin_of_replication origin of replication], and are thus one of the first genes to be replicated [[#References|[2, 9]]]. | ||
Genes required for DNA alkylation [http://en.wikipedia.org/wiki/Alkylation alkylation], recombinatorial and nucleotide excision repair, and SOS response [http://en.wikipedia.org/wiki/SOS_response SOS response] are also present [[#References|[2, 3]]]. These genes aid A. borkumensis in dealing with the damaging effects of UV light it encounters as a result of inhabiting the upper layers of aquatic environments. | Genes required for DNA alkylation [http://en.wikipedia.org/wiki/Alkylation alkylation], recombinatorial and nucleotide excision repair, and SOS response [http://en.wikipedia.org/wiki/SOS_response SOS response] are also present [[#References|[2, 3]]]. These genes aid A. borkumensis in dealing with the damaging effects of UV light it encounters as a result of inhabiting the upper layers of aquatic environments. | ||
| Line 55: | Line 53: | ||
<br> | |||
=Metabolism= | =Metabolism= | ||
| Line 65: | Line 59: | ||
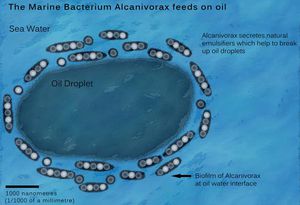
A. borkumensis can use n-alkanes, aliphatic hydrocarbons, volatile fatty acids and pyruvate as carbon and energy sources [[#References|[6]]]. When using only n-alkanes as carbon and energy sources, A. borkumensis produces extracellular and membrane-bound glucose lipids termed biosurfactants [[#References|[10]]]. Biosurfactants are crucial to the biodegradation of oil because they reduce surface tension of water and act as natural emulsifiers to elute oil out of water, thereby making it available to biodegrade. | A. borkumensis can use n-alkanes, aliphatic hydrocarbons, volatile fatty acids and pyruvate as carbon and energy sources [[#References|[6]]]. When using only n-alkanes as carbon and energy sources, A. borkumensis produces extracellular and membrane-bound glucose lipids termed biosurfactants [[#References|[10]]]. Biosurfactants are crucial to the biodegradation of oil because they reduce surface tension of water and act as natural emulsifiers to elute oil out of water, thereby making it available to biodegrade. | ||
<br> | |||
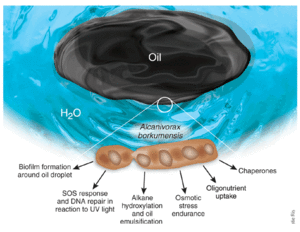
[[File:eating.jpg|300px|thumb|left| A depiction of the mechanism by which A. borkumensis degrades oil droplets [7F] ]] | |||
<br> | |||
Although little is known about the exact mechanism used by A. borkumensis to biodegrade oil, a hypothesis summarizes the method with the following steps [[#References|[6, 9]]]: | Although little is known about the exact mechanism used by A. borkumensis to biodegrade oil, a hypothesis summarizes the method with the following steps [[#References|[6, 9]]]: | ||
1.) oil leakage into aquatic environments causes an increase in phosphorus and nitrogen concentrations | 1.) oil leakage into aquatic environments causes an increase in phosphorus and nitrogen concentrations | ||
| Line 74: | Line 70: | ||
5.) Biosurfactant produced and breaks oil and water emulsions to make oil more readily available for A.borkumensis | 5.) Biosurfactant produced and breaks oil and water emulsions to make oil more readily available for A.borkumensis | ||
<br> | |||
<br> | |||
<br> | |||
=References= | =References= | ||
Latest revision as of 01:56, 13 August 2013
Classification
Domain: Bacteria Phylum: Proteobacteria Class: Gammaproteobacteria Order: Oceanospirillales Family: Alcanivoracaceae Genus: Alcanivorax Species: borkumensis
Description
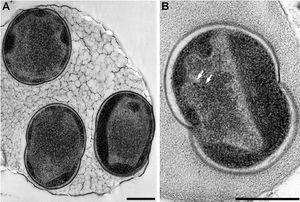
Alcanivorax borkumensis (A. borkumensis) is a Gram-negative, rod-shaped hydrocarbonoclastic (“oil-degrading”) bacterium. It thrives in halophilic, aerobic environments and is found in the upper layers of freshwater or marine environments such as the Mediterranean Sea, Pacific Ocean and Arctic Sea [4, 8]. This bacterium is significant because it is involved in the bioremediation of oil-contaminated aquatic environments [1].
In unpolluted water, A. borkumensis is found in low numbers. This is due to the limited availability of nutrients, namely phosphorus and nitrogen. However, in oil-contaminated water, the addition of phosphorus and nitrogen allows A. borkumensis populations to thrive [2, 4].
Ecology and Significance
Under optimal conditions, A. borkumensis can be the dominant species present within a contaminated area and can comprise up to 80% to 90% of the oil-degrading microbial population present [2]. An example illustrating the significance of A. borkumensis is the Enbridge oil spill that occurred in July 2010 in Michigan’s Kalamazoo River Enbridge Oil Spill. Close to 900,000 gallons of crude oil were leached into the water due to a ruptured pipeline. Over time, scientists discovered A. borkumensis populations to flourish and the degradation of crude oil to be accelerated [11]. If not in part to the metabolism of A. borkumensis (see subsection 3), the decimation of wildlife and marine populations would have been rampant.
A. borkumensis is a native species and is adapted to living in oil-contaminated aquatic environments [2, 4]. Its genome encodes for a broad spectrum of efficient oil-degrading enzymes that can be used in bioremediation of oil spills [3, 6, 9]. This provides A. borkumensis with a competitive advantage in that it can consume a wider variety of alkanes than other known species and thus become the dominant population in an oil-contaminated area [2]. Research is now underway to manipulate the growth of A. borkumensis with amendments of phosphorus and nitrogen fertilizers [2, 4]. Scientists are also looking to isolate genes encoding oil-degrading systems and insert these into a novel organism that can be used to remediate oil spills. However, there are risks associated with each of these methods. The addition of phosphorus and nitrogen in the form of fertilizer may actually contaminate aquatic environments further and aggravate environmental degradation [2, 3]. With respect to genomic transfer, there is the possibility of rapid DNA evolution molecular evolution which can result in harmful, dominant bacteria that can choke other existing species in aquatic environments [2, 3].
Genome Structure
A. borkumensis strain SK2 is the first hydrocarbonoclastic bacterium to have its genome sequenced genome sequencing [7]. The genome is comprised of a single circular chromosome with an average G+C content GC-content of 54.7% [3, 7]. Genomic analysis revealed the importance of three main systems [7]: a.) Degradation of n-alkanes: metabolism, biosurfactant surfactant and biofilm biofilm production b.) Acquisition of phosphorus, nitrogen, sulfur and other elements to allow for alkane degradation c.) Tolerance to stress factors such as high UV radiation encountered in its natural habitat of top layers of aquatic environments
As with all vital systems of microorganisms, enzymes are the critical factors which support the function of these three main systems. A. borkumensis produces the enzymes AlkB1 and AlkB2 [9]. Both are alkane hydroxylases hydroxylation but AlkB1 oxidizes linear or branched alkanes in the range of five to twelve carbons, whereas AlkB2 oxidizes linear or branched alkanes in the range of eight to sixteen carbons. The importance of AlkB1 and AlkB2 can be appreciated by the fact that both genes encoding the enzymes are located close to oriC origin of replication, and are thus one of the first genes to be replicated [2, 9].
Genes required for DNA alkylation alkylation, recombinatorial and nucleotide excision repair, and SOS response SOS response are also present [2, 3]. These genes aid A. borkumensis in dealing with the damaging effects of UV light it encounters as a result of inhabiting the upper layers of aquatic environments.
Metabolism
A. borkumensis can use n-alkanes, aliphatic hydrocarbons, volatile fatty acids and pyruvate as carbon and energy sources [6]. When using only n-alkanes as carbon and energy sources, A. borkumensis produces extracellular and membrane-bound glucose lipids termed biosurfactants [10]. Biosurfactants are crucial to the biodegradation of oil because they reduce surface tension of water and act as natural emulsifiers to elute oil out of water, thereby making it available to biodegrade.
Although little is known about the exact mechanism used by A. borkumensis to biodegrade oil, a hypothesis summarizes the method with the following steps [6, 9]: 1.) oil leakage into aquatic environments causes an increase in phosphorus and nitrogen concentrations 2.) increased nutrient availability causes A.borkumensis to metabolize and grow faster; population increases 3.) A.borkumensis attaches and forms a biofilm around the oil droplet. The biofilm aids in the recruitment of additional bacteria to the site of contamination 4.) AlkB1 and AlkB2 enzymes synthesized and are used to oxidize C-alkanes, thereby catalyzing the degradation of oil 5.) Biosurfactant produced and breaks oil and water emulsions to make oil more readily available for A.borkumensis
References
[1] Golyshin, Peter N. “Genome Sequence Completed of Alcanivorax borkumensis, a Hydrocarbon-degrading Bacterium That Plays a Global Role in Oil Removal from Marine Systems.” 3 (2003): 215-20. Print.
[2] Hara, Akihiro, Kazuaki Syutsubo, and Shigeaki Harayama. "Alcanivorax Which Prevails In Oil-contaminated Seawater Exhibits Broad Substrate Specificity For Alkane Degradation." Environmental Microbiology 5.9 (2003): 746-753.
[3] Kasai, Y et al. "Predominant Growth of Alcanivorax Strains in Oil-contaminated and Nutrient-supplemented Sea Water." Environmental Microbiology 4.3 (2002): 141-47.
[4] Martins, VAP et al. "Genomic Insights into Oil Biodegradation in Marine Systems." Microbial Biodegradation: Genomics and Molecular Biology (2008). Caister Academic Press
[5] Reva, Oleg N. “Global Features of the Alcanivorax Borkumensis SK2 Genome.” 10.3 (2007): 614-25. Web.
[6] Sabirova, Julia S. “Proteomic Insights into Metabolic Adaptations in Alcanivorax Borkumensis Induced by Alkane Utilization.” 188.11 (2006): 3763-773. Web.
[7] Schneiker, Susanne et al. "Genome Sequence of the Ubiquitous Hydrocarbon-degrading Marine Bacterium Alcanivorax Borkumensis." Nature Biotechnology 24.8 (2006): 997-1004.
[8] Timmis, Kenneth N. “Obligate Oil-degrading Marine Bacteria.” 18.3 (2007): 257-66. Web
[9] Van Beilen, J. B. et al. “Characterization of two alkane hydroxylase genes from the marine hydrocarbonoclastic bacterium Alcanivorax borkumensis.” Environmental Microbiology 6 (2004): 264–273.
[10] Yakimov, Michail M. “Alcanivorax Borkumensis Gen. Nov., Sp. Nov., a New, Hydrocarbon-degrading and Surfactant-producing Marine Bacterium.” 48.2 (1998): 339-48. Web.
[11] Siegel, R. P. "Evidence Mounts of Continued Harm from the Gulf Spill." Triple Pundit RSS. N.p., 20 Sept. 2010. Web. 22 Nov. 2012. <http://www.triplepundit.com/2010/09/evidence-mounts-of-continued-harm-from-the-gulf-spill/>.
Figures
[1F]
[1] [2F] [2] [3F] [3] [4F] [4] [5F] [Original Figure. Author: Pawan Dhaliwal] [6F]
http://microbewiki.kenyon.edu/index.php/File:Lorenzo.gif
[7F] [5]