Anchialine pools and cenotes
Introduction
Anchialine pools, also known as Cenotes in the Yucatan Peninsula region, are subterranean bodies of water that have a connection to the surface, some with connections to the ocean. Anchialine pools are circular and cliffed sinkholes that contain watertable lakes (Webb et al. 2009). They are filled with brackish water. They contain large passage ways which make them desirable for cave explorations (Brock and Bailey-Brock 2007). They are very common globally, with the most popular located in the Hawaiian Islands and the Yucatan Peninsula, Mexico. They are also located in the Florida peninsula, southeastern South Australia, South Africa, Turkey on the Anatolian Plateau, and the Bahamas Banks. In the Bahamas Banks, they occur onshore and offshore, where they occur as drowned anchialine pools called Blue Holes (Donachie et al. 2004). Sistema Zacaton, Mexico, is the world's largest known cenote that is over 300 meters deep (Fairfield et al. 2009).
Anchialine pools serve as the main source of water for humans and animals in regions like the Yucatan Peninsula (Schmitter-Soto et al. 2002). They are home to many endemic flora and fauna, like crustaceans and fishes (MacSwiney et al. 2007). Since the water in these lakes is so clear, they are prime places for scuba diving and cave diving. Both of these activities also have the potential to harm the ecosystems that occur in cenotes. Anchialine pools are threatened by nitrate contamination from untreated animal wastes, leaching of fertilizers, vegetation removal and invasive species (Schmitter-Soto et al. 2002).
Chemical Environment
Anchialine pools have many different parameters. Some of these parameters include temperature, pH, and total organic carbon. Cenotes contain many different anions: PO 43-, Cl-, NO3-, Br, HCO3-, and SO42-. Cations that exist in cenotes are Ca, K, Mg, Na, Si, and Sr. Trace elements are Zn, Mo, As, and Cd. They also include NH4+, H2S, and S (Sahl et al. 2011). Cenotes have a high sulfur content and lack sunlight or dissolved oxygen. Sulfate-reducing bacteria, like Desulfovibrio spp., reduce sulfate (SO42-) into sulfide gas (H2S) (Fairfield et al. 2009). The mixing zone between fresh and salt water is a chemically aggressive solution and accelerates the dissolution of limestone (Pohlman 2011).
Chemosynthesis occurs within anchialine systems. Hydrogen sulfide is produced by sulfate reduction of organic matter. The hydrogen sulfide that is produced can then be used as an electron source for chemolithoautotrophs. Redox gradients develop in anchialine pools within the water column where marine water and freshwater mix. Stable carbon isotope data has been used to delineate chemosynthetic and photosynthetic organic matter contributions and then track those through the food web. Both chemosynthesis and photosynthesis fix carbon dioxide in anchialine pools. Chemosynthesis can be the primary source of energy in some cenotes (Pohlman 2011).
Physical environment
Vegetation
There are many plant species that are endemic to anchialine pools. Characteristic heterogeneous vegetation, including tall evergreen trees like Ficus species, surrounds the opening of the pools. Floating macrophytes inhabit part of the surface water in oligotrophic anchialine pools. Removal of vegetation by large herbivores is threatening the ecosystems that reside in the anchialine pools. Threats to anchialine pools include sedimentation, altered hydrologic processes, and nutrient filtering when vegetation is removed (MacSwiney 2007).
Geology
Anchialine pools are common on coastal karst plains with low topography. Typically karst landscapes are limestone, dolomite, or gypsum. (Sahl et al. 2011). Anchialine pools are formed by the dissolution of limestone by carbonic acid in karst landforms. Underlying these landforms is calcerous rock. As the rock breaks off and falls into the pool, it is further dissolved. The cracks eventually create anchialine pools, or sinkholes, in the surface as the rock further weathers. In the Hawaiian Islands, these pools occur in h ighly porous substrates like recent lava flows or limestone that are near the ocean (Brock and Bailey-Brock 2007). Inputs of organic matter are received in different ways. Fully marine caves and anchialine tubes get organic matter from the coastal ocean. Inland anchialine caves receive terrestrial organic matter from sources thousands of meters from the coast and chemoautotrophic organic matter sources. Terrestrial organic matter sources include: surface soils, paleosols, and decaying plants as well as aquatic vegetation (Pohlman 2011).
Hydrology
Anchialine pools experience tidal fluctations and lack surface connections that are near the ocean. They contain very clear water and large channels. The anchialine pools of the Yucatan Peninsula in Mexico are the only known underground aquatic system located there. They are stratified, with brackish water on the top layer. They experience a salinity gradient throughout the water column (Mejia-Ortiz et al. 2007). The mixing between meteoric freshwater and coastal marine water is a prominent hydrologic feature of anchialine pools this creates large salinity differences. The differences between salinity and temperature create separate the water column (Pohlman 2011).
Microbial communities
Anchialine pools contain a high concentration of chlorophyll which indicates the presence fo algae such as chlorophyceans, cyanobacteria, diatoms, and dinoflagellates. The hypolimnion and sediment have high amounts of organic matter in which anaerobic bacteria and archaea thrive. Anchialine pools are considered heterotrophic systems because they contain such high amounts of organic matter input and production and low water flow. Anchialine pools are home to different types of microbes. Thick and intricate microbial mats also form in these areas due to nutrients provided by hydrothermal inputs. The microbes in the microbial mats undergo anaerobic respiration. Microbial processes that occur here include: methanogenesis, sulfate reduction, and anaerobic ammonia oxidation.
Microbial Mats
Microbial mats cover the upper part of anchialine pool walls. These mats form stratified, complex communities comprised mostly of bacteria and archaea. The mats can range in size from a few millimeters to a few centimeters. Microbial mats have distinct layering of different microbial populations. Some common microbes found in anchialine pools in Mexico include Epsilonproteobacteria, Betaproteobacteria,Chlorobi, and Chlorophycea. Microbial communities change throughout the water column. There is a phylogenetic distribution shift between shallow and deep water-column areas. Surface samples were dominated by Gammaproteobacteria, Rhodocyclales, and Neisserales. As water depth increases, Bacteroidetes can be observed, as well as Gammaproteobacteria. Deltaproteobacteria, Nitrospirae and Chloroflexi can be observed at depth within microbial mats. Archaea are found in microbial mats in cenotes including methanogens (Methanomicrobia) and anaerobic methane oxidizers (ANME-1)(Fairfield et al. 2009). Cyanobacteria can also be found at shallow depths in cenotes (Sahl et al. 2011). Cenotes in Hawaii have a somewhat different composition: marine green sulfur bacteria Chlorobium vibrioforme and Prosthecochloris aestuarii, iron-oxidizing bacteria Leptothrix cholodnii, Marinobacter sp. (a deep-sea iron-oxidizer. Other microbes included: Deinococci, Planctomycetes, Fibrobacter/Acidobacter, Verrucomicrobia, Cyanobacteria, and Euryarchaeota (Donachie et al. 2004).
Calothrix Mat
This is the most common type of microbial mat. It is made of upward tapered filaments of the rivulariacean cyanobactera Calothrix. The mat forms shores at low angles. The filaments of Calothrix are branched to form upward diverging bushs that give the stromatolite interior of the cenote. This type of microbe forms heterocysts. Calothrix mats have the widest distribution in the lake. Other bacteria associated with this mat include Schizothrix, Scytonema, Entophysalis, and Gloeothece (Gischler et al. 2011).
Scytonema Mat
These microbial mats form around habitats with strong currents. These mats are almost always entire populations of Scytonema sp. Structures are dome-shaped and covered by a biofilm with calcified sheaths. Filaments are curved and intertwined but are not oriented upwards (Gischler et al. 2011).
Leptolyngbya Mat
These mats are bright orange in color and grow on top of microbialite heads. They are very close to the surface of the water and are partially exposed to the atmosphere. There are produced by cyanobacteria with narrow trichomes known as Leptolyngbya. they consist of unbranched cellular trichomes surrounded by a thin sheath. The orange color the the mats reflect high concentrations of carotenoids in the cells, this protects microbes underneath the mat from UV radiation. Underneath this protection layer, the mat is blue-green in color (Gischler et al. 2011).
Methanogens
Methanogens (Methanomicrobia) are a type of microbe that belongs to the domain Archaea. They undergo a type of anaerobic respiration called methanogenesis.
Sulfate reducing bacteria
Sulfate reducing bacteria are obligate anaerobes that convert sulfate (SO42-) to hydrogen sulfide gas (H2S) Specific bacteria that reduce sulfur include Desulfovibrio spp., Desulfomonas spp., and Desulfotomaculum spp. These species of bacteria use end products of other fermentations like lactate, malate, and ethonal as electron donors.
Ammonium oxidizing bacteria
Ammonium oxidizers are anaerobic microbes that undergo ammonium oxidation. Nitrifying microorganisms include both bacteria and archaea. There include microbes that oxidize ammonia (NH3) and those that oxidize nitrite (NO2-) directly into dinitrogen gas. Ammonia (NH3) oxidizers include members from the betaproteobacteria group (Nitrosomonas spp., and Nitrosospira spp.) and from the gammaproteobacteria group (Nitrosococcus spp.). Nitrite (NO2-) oxidizers include members from the alphaproteobacteria group (Nitrobacter spp.), gammaproteobacteria group (Nitrococcus spp.), deltaproteobacteria group (Nitrospina spp.), and Nitrospira spp.
Microbial processes
Anaerobic respiration
Anaerobic respiration occurs when microbes use other terminal electron acceptors other than oxygen. In environments, like anchialine pools and cenotes, microbes use terminal electron acceptors like sulfate (SO4-2), nitrate (NO3-), sulfur (S), and carbon dioxide (CO2) (Fairfield et al. 2009). This type of respiration occurs in the absence of oxygen. Although these terminal electron acceptors produce energy, they release less energy than oxygen. Therefore, anaerobic respiration produces less energy than aerobic respiration.
Methanogenesis
Methanogenesis is the formation of methane by microbes. Methanogens, a type of Archaea, specifically carry out this process. This is a type of anaerobic respiration that uses carbon dioxide (CO2) and acetic acid as the terminal electron acceptors.
CO2 + H2 → CH4 + 2H
CH3COOH → CH4 + 2H
Sulfate reduction
Sulfate (SO42-) is part of the sulfur cycle. Sulfate reduction occurs when sulfate is reduced to sulfide (S-). Sulfate-reducing bacteria use sulfate as the terminal electron acceptor in the anaerobic conditions that occur in anchialine pools. This is especially important in the water-logged sediments in the benthos of these pools (Fairfield et. al 2009).
Sulfide oxidation
Sulfide oxidation occurs when microbes oxidize sulfide (S2-) or hydrogen sulfide gas (H2S) and producing sulfate (SO42-). Microbes produce electrons and make ATP (Sylvia et al. 2005).
Fermentation
Fermentation occurs in cenotes where oxygen is not available. Iron-iron hydrogenase sequences associated with fermentation in bacteria have been amplified from shallow water samples of cenotes (Sahl et al. 2011).
Ammonia oxidation
Ammonia (NH3) oxidation occurs when ammonia is oxidized into nitrite (NO2-). There are three different ammonia oxidizers groups: Nitrosomonas, Nitrosospira, and Nitrosococcus. This is an endergonic reaction and requires a small amount of energy. Ammonia monooxygenase facilitates this reaction. Ammonia oxidation produces nitrous oxide (N2O) and acidity. Ammonia oxidation can cause acidity in anchialine pools (Sylvia et al. 2005).
Nitrite oxidation
Nitrite oxidation is the final step in ammonia oxidation. Nitrobacter spp. and Nitrospira spp. can oxidize nitrite (NO2-) into nitrate (NO3-). Nitrite oxidoreductase facilitates this reaction (Sylvia et al. 2005).
Lithotrophic Processes
Lithotrophic processes occur when lithotrophs utilize inorganic substrates to gain energy. A variety of microbes can do this including ammonium oxidizing-bacteria and archaea.
Anaerobic ammonia oxidation
Aerobic ammonium oxidation is part of the nitrogen cycle. Ammonium-oxidizing bacteria convert nitrite (NO2-) and ammonium (NH4+) directly to nitrous oxide ((N2O) (Sahl et al. 2011). All ammonia oxidizers contain nitrite reductase which can reduce nitrite directly into nitrous oxide. Production of nitrous oxide occurs more often hen oxygen availability decreases because nitrite is being used as a terminal electron acceptor (Sylvia et al. 2005).
NH4+ + NO2- → N2 + 2H2O
Current Research
"A Comparative Molecular analysis of Water-filled Limestone Sinkholes in North-eastern Mexico"
Researchers are using new methods to study cenotes in Sistema Zacaton in north-eastern Mexico. Microbial mat communities were studied using comparative analysis of small-subunit 16S rRNA gene sequences. Genes associated with methanogenesis, sulfate reduction and anaerobic ammonium oxidation were also identified and studied (Sahl et al. 2011).
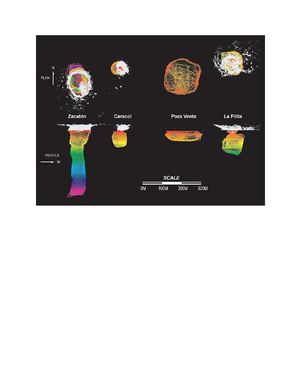
"Autonomous Exploration and Mapping of Flooded Sinkholes"
NASA conducted an experiment in which flooded cenotes were mapped using the DEPTHX (Deep Phreatic Thermal Explorer) autonomous vehicle in Sistema Zacaton in Mexico. Three-dimensional maps of cenotes were constructed and environmental data, imagery, water samples, and core samples were taken from the cenotes in this region. Four different cenotes were studied and mapped: La Pilita, Zacaton, Verde, and Caracol (Fairfield et al. 2010).
"Volcanogenic origin of cenotes near Mt Gambier, southeastern Australia"
Researchers studied the origin of cenotes in southeastern Australia. Caves had collapsed to form the Mt Gambier cenotes. Acidified groundwater containing significant amounts of volcanogenic carbon dioxide that has ascended up cracks from the magma chambers formed the cenotes in this area, as opposed to how most cenotes are formed by freshwater/seawater mixing. This research shows that there are different ways that cenotes form and further investigation needs to occur to fully understand the formation of these sinkholes (Webb et al. 2010).
References
Gischler, E., Golubic, S., Gibson, M.A., Oschmann, W., and hudson, J.H. "Microbial mats and microbialites in the freshwater Laguna Bacalar, Yucatan Peninsula, Mexico". Advances in Stromatolite Geobiology. 2011. Volume 131. p. 187-205.
Pohlman, J.W. "The biogeochemistry of anchialine caves: progress and possibilities". Hydrobiologia. 2011.
Sylvia, D.M. Fuhrmann, J.J., Hartel, P.G., and Zuberer D.A. Principles and Applications of Soil Microbiology 2nd Edition. New Jersey: Pearson Prentice Hall, 2005. Print.
Edited by Lauren Behnke, a student of Angela Kent at the University of Illinois at Urbana-Champaign.