Arthrospira platensis
A Microbial Biorealm page on the genus Arthrospira platensis
Classification
Higher order taxa
Domain: Bacteria
Phylum: Cyanobacteria
Class: Cyanophyceae
Order: Oscillatoriales
Family: Phormidiaceae
Species
Genus: Arthrospira
Species: platensis
Arthrospira (Spirulina) platensis; A. platensis
Description and significance
Arthrospira platensis, also known as Spirulina, is a gram negative, non-toxic species of cyanobacteria with a wide array of uses in the natural and commercial world. While many bacteria are known for their pathogenic effects, A. platensis is primarily known across the world for its potential nutritional value. It is one of the rare edible bacteria due to its low purine concentration, which allows it to pose very minimal risk of uric acid build up in the body(12). Historically, it is known to have been regularly consumed by the Aztecs and tropical climate populations where it was often dried into flat patties for easy consumption(3). Because of its anti-carcinogen properties, it was also used to treat radiation sickness in people that were affected by the 1986 Chernobyl nuclear accident(9). More recently, the consumption of this species has been shown to lower blood pressure and reduce cholesterol, which are two of the most prevalent health concerns in the modern world(10). The food industry classifies A. platensis as a single-celled protein, meaning that it is an edible microbe with a high food value(12). It is rich in vitamins, minerals, beta-carotene, essential fatty acids, and antioxidants, all of which have facilitated its commercial production as a human food supplement over the course of the past decade(13). It also has very high protein content with a well-balanced composition of all essential amino acids, making it even more desirable as a food supplement(9).
Genome structure
In March 2010, Arthrospira platensis became the first filamentous, non-N2-fixing cyanobacterium to have its genome sequenced and published(3). The genome consists of a single, circular chromosome and was found to be 6.8Mb in size with 44.3% G-C content(3). There were 6630 protein-coding genes detected, as well as 49 RNA genes, including 2 sets of rRNA genes and 40 tRNA genes(3). When looked at in its entirety, 78% of the species' genes showed similarity to genes with known function in other organisms, while 22% of the genome is made up of unknown genes(3).
Cell structure and metabolism
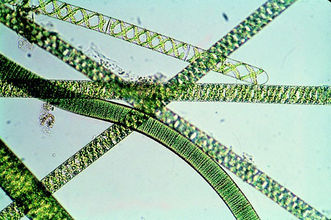
A. platensis is a multicellular, filamentous cyanobacterium. Each unbranched, cylindrical filament of cells is called a trichome and is approximately 5 micrometers in diameter. These trichomes vary greatly in length and are typically spiral-shaped, though they can take on a left-hand helical structure in liquid media(2). Individual cells within the filament are wider than they are long and are separated by transverse cross-walls. The cell wall contains 4 layers: an innermost fibril layer, a second peptidoglycan layer, a third layer composed of proteins, and an outermost layer analogous to the cell wall of all gram-negative bacteria(2). Although A. platensis does not possess any flagella, it has been observed as having a gliding motility, which is still poorly understood(2).
The cells of this cyanobacteria also contain Na+/H+ antiporters which give them resistance to high salt concentrations(6), and they develop cAMP-dependent signal cascades to adapt to severe environmental conditions(3). Both of these adaptations are instrumental in the cells’ ability to survive in the high salinity lakes where this bacteria thrives.
Dispersal of A. platensis is achieved through the production of hormogonia. These are short, motile segments of 3-5 cells that break off of the filament and scatter(12). Hormogonia are also a means of asexual reproduction.
A. platensis is photoautotrophic, obtaining energy from sunlight and converting carbon dioxide and water into sugars. The most important factor that governs A. platensis's growth is the presence of light. Interestingly, strains of the species grown at suboptimal temperatures have been shown to be more sensitive to photoinhibition than those grown at optimal temperatures(4). Its cells contain chlorophylls, carotenoids, and phycobiliproteins, which are pigments capable of absorbing light energy and funneling it to the reaction centers where photosynthesis takes place. The cytoplasm of A. platensis also contains gas vacuoles, carboxyzomes, and thykaloid membranes as adaptations to being phototrophic. The gas vacuoles within the cells increase the cell’s buoyancy so that it remains at the surface of its aqueous environment where the presence of light is greatest. The carboxysomes are protein-enclosed compartments that contain the enzyme, Rubisco, necessary for fixing carbon dioxide, and the thylakoid membranes contain the pigments previously mentioned.
Many cyanobacteria also have the capability of fixing Nitrogen due to the development of heterocysts, which are specialized cells within the bacteria filaments that exclude oxygen and sustain a reduced environment(12). However, A. platensis lacks these structures and can, therefore, not complete the process of Nitrogen fixation.
Ecology
A. platensis inhabits tropical and subtropical bodies of water with high pH (8-11) as well as high levels of carbonate and bicarbonate. The preference of an alkaline marine environment means that this bacteria can be classified as an alkaliphile. It is a free-floating species that tends to aggregate and form mats along the periphery of the aqueous environment. It is found mainly in African lakes, such as Lake Magadi, but also in Asian and South American regions(4). Aside from being found in naturally in these areas, it is also grown commercially for its use as a food additive(10). The bacteria are typically grown for the food industry in large, lined pools that can either have direct access to light or are placed under large lamps. Conditions for optimal growth of A. platensis have been analyzed and include standard mineral nutrient, a temperature range of 30-34 degrees C, pH range of 8.5-11, and a sodium light lamp(9). The maximum growth of the species is achieved on the fourth or fifth day of cultivation(9).
Pathology
A. platensis is not pathogenic in nature. On the contrary, it has been shown to potentiate the immune system of its human hosts. It is believed that this microorganism suppresses both cancer development and viral infection (4).
Current Research
Scientists in Japan isolated Calcium spirulinan, abbreviated as Ca-SP, from Spirulina platensis. They investigated the effect of this sulfated polysaccharide chelating calcium on cancer cells, including melanoma, carninoma, and fibrosarcoma cells. The paper was titled the “Inhibition of tumor invasion and metastasis by calcium spirulan (Ca-SP), a novel sulfated polysaccharide derived from a blue-green alga, Spirulina platensis.” Researchers discovered that through IV injections, Ca-SP both decreased metastasis of melanoma cells in the lung, as well as decrease tumor colony growth(8).
In 2002, scientists from Denver, Colorado and Tampa, Florida tested high-antioxidant foods on aged brain function. The foods tested were cucumber, apple, and spirulina. The research paper was titled “Diets Enriched in Foods with High Antioxidant Activity Reverse Age-Induced Decreases in Cerebellar β-Adrenergic Function and Increases in Proinflammatory Cytokines.” Cucumber was found to have no effect. Spirulina had the strongest results out of all three test subjects(5). Rats that were fed spirulina had improved β-adrenergic receptor function, down regulation of pro-inflammatory cytokines, and decreased MDA in the cerebellum(5).
In 2009, researchers in Mexico tested the effect of Spirulina on vascular reactivity. Previous to this study, the benefits of Spirulina were known to be positive against cardiovascular diseases, but this study specifically studied its effect on plasma lipids and blood pressure. Results showed that Spirulina decreased blood pressure and lipid concentrations in plasma (7). It also decreased LDL levels (low-density lipoproteins) while indirectly increasing HDL levels (high-density lipoproteins), providing yet another potential health benefit of this processed bacteria (7).
Cool Factor
This cyanobacteria is considered a ‘super food’ by many scientists. According to a literature review researched by Cyanotech Corporation, Spirulina has “180% more calcium than whole milk, 670% more protein than tofu, 3100% more beta carotene than carrots, 5100% more iron than spinach, and more antioxidant and anti-inflammatory activity in 3 g of Spirulina than in five servings of fruits and vegetables(1).”
A. platensis is also the main diet component of flamingos and is responsible for the characteristic pink color of these birds. The abundant carotenes are released from the bacteria as they are digested and are distributed to the feathers, producing a pink hue(12). The color change does not occur in humans who ingest the bacteria as a supplement because it makes up such a small portion of the diet(12).
References
1. Capelli B, Cysewski GR. “Potential Health Benefits of Spirulina microalgae: A review of the existing literature”. Cyanotech Corporation. 9.2 (2010).
2. Ciferri O. "Spirulina, the edible microorganism." Microbiol Rev. 47.4 (1983):551-578.
3. Fujisawa, Takatomo et al. “Genomic Structure of an Economically Important Cyanobacterium, Arthrospira (Spirulina) platensis NEIS-39.” DNA Research 17 (2010): 85-103.
4. Gao, Kunshan, Ma, Zengling. “Photosynthesis and growth of Artropira (Spirulina) platensis (Cyanophyta) in response to solar UV radiation, with special reference to its minor variant.” Environmental and Experimental Botony 63.1-3 (2007): 123-129.
5. Gemma C, et Al. “Diets Enriched in Foods with High Antioxidant Activity Reverse Age-Induced Decreases in Cerebellar β-Adrenergic Function and Increases in Proinflammatory Cytokines”. J. Neurosci. 22.14 (2002): 6114-6120.
6. Hirahashi, Tomohiro et al. “Activation of the human innate immune system by Spirulina: augmentation of interferon production and NK cytotoxicity by oral adminstration of hot water extract of Spirulina platensis.” International Immunopharmacology 2.4 (2002): 423-434.
7. Juarez-Oropeza MA, et Al. “Effects of Spirulina on vascular reactivity.” J Med Food. 12.1 (2009): 15-20.
8. Mishima T, et Al. “Inhibition of tumor invasion and metastasis by calciumspirulan (Ca-SP), a novel sulfated polysaccharide derived from a blue-green alga, Spirulina platensis”. Clinical and Experimental Metastasis. 16.6 (1998): 541-550.
9. Mosulishvili, L. M., E. I. Kirkesali, A. I. Beiokobylsky, and A. I. Khizanishvili. “Experimental Substantion of the Possibility of Developing Selenium and Iodine Containing Pharmaceuticals Based on Blue-green Algae Spirulina platensis.” Journal of Pharmaceutical and Biomedical Analysis 30.1 (2002): 87-97. Web. 5 Oct. 2011.
10. National Center for Biotechnology Information. Web. 05 Oct 2011. <http://www.ncbi.nlm.nih.gov/bioproject?Db=genome&Cmd=ShowDetailView&TermToSearch=6605.>
11. Singh, Nirbhay Kumar and Dolly Wattal Dhar. “Phylogenetic Relatedness Among Spirulina and Related Cyanobacterial Genera.” World J Microbiol Biotechnol 27 (2011): 941-951.
12. Slonczewski, Joan L. and John W. Foster. Microbiology: An Evolving Science. 2nd Ed. New York: W. W. Norton & Company, Inc., 2009. 141-685.
13. Watunuki, Hironubu, Kazuki Ota, Asmi Citra Malina, A. R. Tassakka, Toshimitsu Kato, and Masahiro Sakai. “Immunostimulant Effects of Dietary Spirulina platensis on Carp, Cyprinus Carpio.” Aquaculture 258. 1-4 (2006): 157-63. Web. 5 Oct. 2011.
Edited by student of Iris Keren NEUF2011