Avian Malaria in Hawaiian Birds: Difference between revisions
No edit summary |
|||
| Line 44: | Line 44: | ||
Every point of information REQUIRES CITATION using the citation tool shown above. | Every point of information REQUIRES CITATION using the citation tool shown above. | ||
== | ==Common impact of <I> P. relict </i> on birds== | ||
While symptoms of P. relictum infection may vary from individual from individual, the infection normally follows a predictable course. After the initial infection, the bird will undergo an acute period lasting 6-12 days (LaPointe et al 2013). The intensity of the infection will then increase. This intensity can be effected by a variety of factors, from the host's immune system to the season (LaPointe et al 2013). At peak intensity, birds will commonly be anorexic (LaPointe et al 2013). Their feathers will be in poor condition, and their body weight will decrease due to the anorexia and other factors (LaPointe 2013) (Atkinson et al 2000). They will often be anemic and have a low hematocrit, as seen in their thin, watery blood (LaPointe 2013). Both the liver and spleen will be enlarged and discolored, due to the large parasite load often found in these organs (Atkinson et al 2000). Avian malaria is normally diagnosed through blood samples (Atkinson et al 2000). A small blood sample will be taken in order to make a blood smear on a slide (Atkinson et al 2000). Once blood smears are fixed, the number of infected red blood cells are counted using microscopy (Atkinson et al 2000). In addition to the common method of looking at infected erythrocytes using microscopy, newer diagnostic techniques are starting to be used (Atkinson et al 2014). One of these techniques is the use of PCR (Atkinson et al 2014). DNA will be extracted from blood samples, and the mitochondrial cytochrome b gene from the parasite will be amplified (Atkinson et al 2014). If this gene is present, it can be concluded that the bird does have avian malaria. In some studies, samples that test positive for the parasite cytochrome b gene will be confirmed through a second PCR that will amplify the ribosomal DNA of P. relictum (Atkinson et al 2014). | |||
Include some current research, with at least one figure showing data.<br> | Include some current research, with at least one figure showing data.<br> | ||
Revision as of 19:09, 24 April 2018
]
Plasmodium
While malaria is most commonly thought of in terms of the few species that infect humans, there are several hundreds of haemosporidian parasites that infect a variety of vertebrate hosts [1]
These parasites are commonly split into five genera: Plasmodium, Hepatocystis, Haemoproteus, Parahaemoproteus, and Leucocoytozoon ([2]. Of these genera, species within the Leucocytozoon, Parahaemorptoeus, and Plasmodium genera have been found to infect avian species [3]. Of these, Plasmodium relictum has been devastating the native birds on the island of Hawaii [4].
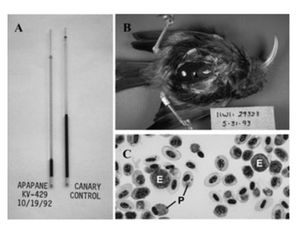
By Jess Kotnour
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Sample citations: [6]
[7]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
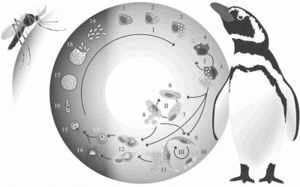
Life Cycle of Plasmodium relictum
Plasmodium relictum follows the common haemosporidian life cycle. P. relictum initially undergoes a period of asexual reprodcution within mosquito host tissues (LaPointe et al 2012). This initial cycle is followed by subsequent cycles of asexual reproduction within both host tissues and red blood cells, leading to gametocytes within host red blood cells (LaPointe et al 2012). These gametocytes will remain in the midgut, becoming gametes until forming fertile zygotes (LaPointe et al 2012). These zygotes will eventually form into oocysts. Inside the oocysts, asexual reproduction will occur to form sporozoties (Atkinson and Van Riper III YEAR). As they rupture from oocysts, these sporozoties will move into the salivary glands of the mosquito, where they can be transmitted intravenously, intramuscularly, or intraperitoneally to birds (Atkinson and Van Riper III YEAR).
Once inside the avian host, the life cycle continues. The sporozoties will invade the avian host’s reticuloendothelial (macrophages and monocytes) cells where they will develop into mernots (Grilo et al. 2016). Through asexual reproduction, mernots will reproduce into merozites while still inside the host’s reticuloendothelial cells (Grilo et al 2016). When the cell inevitably ruptures, the merozites will be released and will spread throughout the host using the blood stream (Grilo et al 2016). P. relictum continues its development in reticuloendothelial and host tissues (Grilo et al 2016). They most commonly develop in the liver, spleen, and kidneys of the host (Grilo et al 2016). Once inside either the host’s cells or tissue, the merozites will further develop into either erythrocytic mernots or gametocytes (Grilo et al 2016). If the merozite develops into a gametocyte, the development is complete, and it will remain in the host’s red blood cells until a mosquito vector bites the host (Grilo et al 2016). If the host is bitten by a mosquito, the gametocyte will repeat the life cycle in the mosquito.
P. relictum often infects birds most often during the breeding months (Atkinson and Van Riper III year). Breeding season is often during the warmer months of the year, allowing for the development mosquitos and P. relictum (Atkinson and Van Ripper III). These mosquitos are able to take advantage of the breeding season, as birds are often investing more resources into raising their young than their immune systems (Atkinson and Van Ripper III Year). The weekend immune system makes infection by P. relictum much easier (Atkinson and Van Ripper III YEAR).
Include some current research, with at least one figure showing data.
Every point of information REQUIRES CITATION using the citation tool shown above.
Common impact of P. relict on birds
While symptoms of P. relictum infection may vary from individual from individual, the infection normally follows a predictable course. After the initial infection, the bird will undergo an acute period lasting 6-12 days (LaPointe et al 2013). The intensity of the infection will then increase. This intensity can be effected by a variety of factors, from the host's immune system to the season (LaPointe et al 2013). At peak intensity, birds will commonly be anorexic (LaPointe et al 2013). Their feathers will be in poor condition, and their body weight will decrease due to the anorexia and other factors (LaPointe 2013) (Atkinson et al 2000). They will often be anemic and have a low hematocrit, as seen in their thin, watery blood (LaPointe 2013). Both the liver and spleen will be enlarged and discolored, due to the large parasite load often found in these organs (Atkinson et al 2000). Avian malaria is normally diagnosed through blood samples (Atkinson et al 2000). A small blood sample will be taken in order to make a blood smear on a slide (Atkinson et al 2000). Once blood smears are fixed, the number of infected red blood cells are counted using microscopy (Atkinson et al 2000). In addition to the common method of looking at infected erythrocytes using microscopy, newer diagnostic techniques are starting to be used (Atkinson et al 2014). One of these techniques is the use of PCR (Atkinson et al 2014). DNA will be extracted from blood samples, and the mitochondrial cytochrome b gene from the parasite will be amplified (Atkinson et al 2014). If this gene is present, it can be concluded that the bird does have avian malaria. In some studies, samples that test positive for the parasite cytochrome b gene will be confirmed through a second PCR that will amplify the ribosomal DNA of P. relictum (Atkinson et al 2014).
Include some current research, with at least one figure showing data.
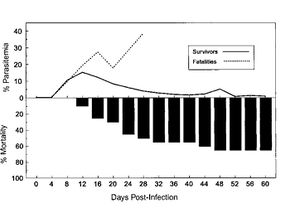
Section 3
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ Martinsen and Perkins 2013. The Diversity of Plasmodium and other Haemosporidians: The Intersections of Taxonomy, Phylogenetics, and Genomics. In: Carlton, J., Perkins, S., Deitsch, editors. Malaria Parasites: Comparative Genomics, Evolution and Molecular Biology. Norfolk: Caister Academic Press. p 1- 15.
- ↑ Martinsen and Perkins 2013. The Diversity of Plasmodium and other Haemosporidians: The Intersections of Taxonomy, Phylogenetics, and Genomics. In: Carlton, J., Perkins, S., Deitsch, editors. Malaria Parasites: Comparative Genomics, Evolution and Molecular Biology. Norfolk: Caister Academic Press. p 1- 15.
- ↑ Martinsen and Perkins 2013. The Diversity of Plasmodium and other Haemosporidians: The Intersections of Taxonomy, Phylogenetics, and Genomics. In: Carlton, J., Perkins, S., Deitsch, editors. Malaria Parasites: Comparative Genomics, Evolution and Molecular Biology. Norfolk: Caister Academic Press. p 1- 15.
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ LaPointe et al.: Ecology and conservation biology of avian malaria. Annals of the New York Academy of Sciences 2012. 1249:211-226
- ↑ Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
- ↑ [https://www.tandfonline.com/doi/full/10.1080/03079457.2016.1149145 Grilo et al.2016. Malaria in penguins—current perceptions. Avian Pathology. 45(4): 393-407. ]
- ↑ Atkinson et al. 2000. Pathogenicity of avian malaria in experimentally infected Hawaii Amakihi. Journal of Wildlife Diseases. 36(2): 197-204
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.
