Bacillus Calmette-Guérin therapy
Summary of the Article
By Karina Kunka
This Microbewiki article is an overview of a type of immunotherapy used to treat superficial and early-stage bladder cancers, and to prevent recurrence thereof. This particular type of immunotherapy utilizes a strain of attenuated Mycobacterium bovis (M. bovis) to stimulate an immune response and cause direct cytotoxicity within the bladder cancer cells. This means that this particular form of cancer treatment is able to use a nonvirulent strain of pathogen to be uptaken by bladder cancer cells; they then cause the bladder cancer cells to express signals to the patient’s immune system that attract phagocytic cells to phagocytose, or break down, the cancerous cells, while preventing damage to healthy tissues. Side effects are very common and often resemble those of a urinary tract infection, or UTI, but typically they are not too severe and can be dealt with using simple pain management strategies such as taking ibuprophen. Bacillus Calmette-Guérin therapy is the most commonly-used form of immunotherapy and remains a treatment of choice for management of early-stage bladder cancers. Its safety and efficacy has been thoroughly researched for multiple decades, and it is generally very effective at preventing recurrence of bladder cancer, especially when maintenance therapy for several months or years is used. The Bacillus Calmette-Guérin strain of M. bovis is also used for a vaccine against tuberculosis infection by Mycobacterium tuberculosis in humans, and important new implications for Bacillus Calmette-Guérin use in other types of cancer treatments are up and coming. This article also explores BCG Therapy in the context of competing theories for its mechanism of action and efficacy as a treatment for bladder cancer in comparison to traditional methods of chemotherapy.
Introduction
Bacillus Calmette-Guérin (BCG) Therapy (Trade name TheraCys®, TICE® BCG)[1] is a form of outpatient intravesical immunotherapy for the treatment of bladder cancer. BCG therapy is typically used to prevent noninvasive or minimally-invasive bladder cancers from returning following transurethral resection of bladder tumor (TURBT)[2] and is widely considered the most effective prophylactic treatment for patients with non-muscle-invasive bladder cancer (NMIBC).[3]
Bacillus Calmette-Guérin is a type of attenuated (weakened or non-virulent) Mycobacterium bovis, depicted at the right in green (Figure 1). It was first cultured at the Pasteur Institute in the early 1900s, where it was then subcultured and distributed worldwide. BCG was first used in a vaccination for tuberculosis infection, then later in the 1980’s adopted for use in immunotherapy as a treatment of bladder cancer. TICE, Armond-Frappier, and Connaught are the only three FDA-approved BCG strains for use in the United States by patients with non-muscle-invasive bladder cancer, but TICE is the only strain actively marketed in the U.S. as of 2017.[4] However, many other strains exist and are used worldwide for the same effect (see: Efficacy).
Mycobacterium bovis
Overview
Mycobacterium bovis is a strain of aerobic, slow-growing bacteria that is the main cause of bovine tuberculosis. It is closely related to Mycobacterium tuberculosis, or the main causative agent of tuberculosis in humans, which can lead to severe infection of the lungs and lymph nodes. M. bovis typically infects cattle, deer, elk, and bison, but is capable of crossing the species barrier and infecting humans with tuberculosis, though it is estimated to cause only 2% of total tuberculosis cases in the United States each year. Transmission is usually through contaminated dairy products or other animal products such as infected meats.[5]
M. Bovis History and Attenuation
For medical use, the virulence of M. bovis must be reduced so as to not lead to infection of the patient during BCG immunotherapy. BCG was first attenuated for use in the BCG vaccine against M. tuberculosis in 1921 by Albert Calmette and Camille Guérin in the Pasteur Institute in Paris, France (See “Other Uses of BCG”). The most common method of attenuation for a bacterial strain is adaptation to a foreign host. The pathogenic bacteria are exposed for periods of time to tissues, eggs, or live specimens of these foreign hosts and allowed to develop mutations that make them better able to survive in the new environment. These mutations make subsequent infection of humans much more difficult, and human pathogenicity of the new strain is decreased. For the creation of BCG from M. bovis, the bacterium was attenuated via serial passage of M. bovis bacteria on glycerol-enriched potatoes.[6] The mechanism of attenuation of BCG is not fully understood, as BCG is not a single strain, but rather a collection of substrains that exhibit differing levels of virulence.[7] However, it is known that attenuated BCG strains contain mutants of several virulence factors of Mycobacterium tuberculosis, such as ESX-1 (a virulence factor secretory system), PDIM/PGL (cell envelope localized Mycobacterium tuberculosis lipids and related glycolipids), and PhoP (a transcriptional regulatory protein), making these mutations important for lack of BCG pathogenesis.[7]
Bladder Cancer
Ranking fourth, bladder cancer is one of the most common types of cancer in men. It was predicted that in the year 2016, almost 77,000 new cases of bladder cancer were responsible for over 16,000 deaths in the U.S. alone.[8] Most cases of bladder cancers present as NMIBC, which includes stages 0 and 1. Ta (non-invasive papillary carcinoma) and CIS (Carcinoma in situ: non-invasive flat carcinoma) make up stage 0, while phase T1 (tumor has grown from bladder lining to connective tissue, but has not yet reached the muscle layer) makes up stage 1 bladder cancer. For these early stages, standard treatment involves removal of papillary lesions with transurethral resection. However, recurrence rates are high even when it appears as if all cancerous tissue is removed, and secondary treatments in the form of BCG therapy are often used.[9]
Delivery and Course of Treatment
BCG therapy is delivered via an intravesicular infusion, meaning that a catheter is inserted into the urethra, and BCG solution is injected directly into the bladder via the catheter, which is then clamped to prevent BCG solution from prematurely exiting the bladder for the duration of the catheterization. The patient receiving BCG treatment must roll side to side and lay in multiple positions in order to guarantee the medication reaches all areas of the bladder. After about 2 hours, the clamped is removed and the fluid is drained, then the catheter is removed. Typically this is repeated on a weekly basis for 6 weeks following TURBT, then maintenance treatment is repeated monthly for a predetermined time by the physician based on risk of recurrence.[1] Current guidelines suggest 1-3 years of maintenance of BCG therapy following TURBT for reducing the chance of recurrence or progression into more aggressive, muscle-invasive forms of bladder cancer.[3] The amount of BCG administered per treatment depends on the manufacturer, the health of the patient, and the cancer type as evaluated by a physician.
Efficacy
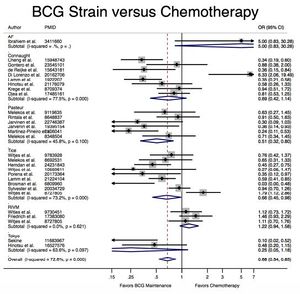
A meta-analysis of 65 studies with a total of 12,246 patients presenting with early-stage bladder cancer receiving Bacillus Calmette-Guérin immunotherapy treatment from 1978-2016 revealed that BCG significantly reduced cancer recurrence when compared with chemotherapy and surgery alone (Figure 2).[4] Recurrence rates varied by strain; for example, the Connaught strain had a 74% 5-year recurrence-free survival rate as compared with the 48% of the TICE strain, and was generally more effective when no maintenance treatments were administered. However, the TICE strain was more effective than the Connaught strain for the time to first recurrence when no BCG maintenance therapy was given. Lack of head-to-head studies makes ranking efficacy difficult, but based on Surface Under the Cumulative Ranking Curve (SUCRA values), the Tokyo, Pasteur, and TICE strains appear to rank 1st, 2nd, and 3rd in efficacy respectively, though no definitive statements can be made.[4]
Mechanism of Action
Overview
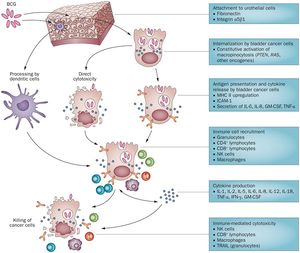
The complete mechanism of action for Bacillus Calmette-Guérin therapy is not fully known, but BCG can be classified as a biological response modifier. This means that the treatment stimulates the immune system to attack the bladder cancer cells, rather than having some sort of primary antitumor capabilities.[1] Currently, Bacillus Calmette-Guérin immunotherapy is hypothesized to be effective through a variety of mechanisms, an outline of which is depicted in Figure 3. The two most commonly-cited mechanisms are direct cytotoxicity and recruitment of immune cells upon infection.
Cells involved
Research suggests that the mechanism of BCG’s antitumor effects involves cells of the immune system and urothelial cells, which includes both cells lining the bladder and the bladder cancer cells themselves. The cancer cells must attach and internalize the BCG cells, secrete cytokines and chemokines, and express BCG antigens for localization by immune cells. Immune cells currently thought to play a role in BCG’s antitumor effects include CD4 and CD8 lymphocytes, as well as many others including dendritic cells, macrophages, granulocytes, and natural killer cells. These immune cells secrete soluble factors to induce direct cytotoxicity such as TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) to kill the BCG- infected bladder cells. There is little debate on the importance of the involvement of these cells, as other studies have found that BCG therapy is ineffective in mouse models deficient in natural killer cell function. However, a 2003 review article stressed the importance of T-helper cells more than in later publications.[10] The direct action of BCG (i.e. cancer cell lysis as a result of internalization of BCG) is also thought to play a role in antitumor effects, if only to some smaller degree.[11]
Uptake of BCG by Bladder Cancer Cells
It would be expected for bladder cancer cells to uptake BCG through phagocytosis, but recent studies have found that the Pak1-dependent pathway activated by oncogenes is actually responsible for uptake of BCG via macropinocytosis. Redelman-Sidi et al. determined that cells vary drastically in their ability to uptake BCG, and bladder cancer cells’ use of micropinocytosis to internalize BCG may account for some of this variability. There are two resulting classes of bladder cancer cells: the Bacillus Calmette-Guérin permissive and resistant cells, with the BCG-permissive cells having a much higher degree of BCG uptake than the resistant phenotype.The proteins responsible for uptake of BCG into bladder cancer cells are Rac1, Cdc42, and Pak1 as an effector kinase. Differences in susceptibility of BCG passive versus resistant cells was found to be caused by oncogenic activation of the signaling pathways responsible for the activation of micropinocytosis. The phosphoinositide 3-kinase inhibitor stimulates GCB uptake independently of Akt. Activated Ras protein also activated Pak1-dependent uptake of BCG. This means that the activation of micropinocytosis by oncogenes determines the degree of BCG uptake by urothelial cancer cells. Furthermore, this may also imply that antitumor effects of BCG may be governed by specific mutations affecting the pathways stated above that are present in the BCG-treated cancer cells. In effect, BCG therapy relies on uptake mechanisms tied to the same mutations that lead to the formation of these cancer cells, meaning that it exploits this property specific to bladder cancer cells to induce an immune response that attacks the cancer instead of normal urothelial cells.[11]
Proposed Step-by-step overview of BCG therapy mechanism of action from attachment to immune cell recruitment
1) Attachment to the urothelial cells is accomplished through the use of fibronectin and integrin α5β1. The bladder cancer cells internalize BCG through constitutive activation of micropinocytosis though oncogenes such as PTEN (Phosphatase and tensin homolog) which acts as a tumor suppressor when functional, RAS (a small GTPase protein involved in cellular signal transduction ), and others.[11] The percentage of cells that actually attach to the bladder wall upon BCG therapy administration is unknown, though estimates from mouse models suggest it is fairly low.
2) Once BCG cells are internalized, they cause either direct cytotoxicity or stimulate presentation of cytokines and antigens. MCH II (a class II antibody) is upregulated, ICAM-1(Intercellular Adhesion Molecule 1) is produced, and interleukin (IL) 6 & 8, GM-CSF (granulocyte macrophage colony-stimulating factor), and TNF- α (Tumor Necrosis Factor-α) are secreted.[11]
3) Bladder cancer cell disruption via direct cytotoxicity or cytokine/antigen presentation results in recruitment of immune cells such as granulocytes, CD4 lymphocytes, CD8 lymphocytes, natural killer cells, and macrophages. The cytokines produced in this process of immune cell recruitment include IL-1, IL-2, Il-5, IL-6, IL-8, IL-12, IL-18, TMF- α, IFN-γ (interferon-γ), and GM-CSF.[11]
4) Immune cell recruitment and cytokine production ultimately results in immune-mediated cytotoxicity by natural killer cells, CD8 lymphocytes, and macrophages. Tumor necrosis factor-related apoptosis-inducing ligand is also secreted by granulocytes to break down the cancer cells.[11]
It is also important to note that not all BCG is taken up by bladder cancer cells. While some BCG is internalized by cancer cells causing cytotoxicity and stimulating immune responses, other BCG cells are broken down by dendritic cells that help protect normal urothelial cells from BCG infection.[11]
Complications of BCG Therapy
Complications of BCG Therapy are widely variable from patient to patient, and can also vary based on dosage and duration of treatment. The most common complications of TheraCys brand BCG treatment, for example, are temporary dysuria (painful or difficult urination), increased frequency and urgency of urination, malaise (fatigue), hematuria (the presence of microscopic or visible amounts of blood in the urine), fever and chills, cycstitis (Urinary Tract Infection, or UTI/ bladder infection that may progress to kidney infection), and nausea. TheraCys reports these more common symptoms as occurring in over approximately 10% of patients, while approximately 50% of patients reported some amount of bladder irritability within 4-6 hours after treatment was administered and lasting for 24-72 hours.[12] Less common symptoms include anemia (low levels of red blood cells) and its associated side effects, nephrotoxicity (issues involving the kidneys), vomiting, and anorexia, or loss of appetite.[1]
In a 2014 study of 1,316 patients with non-muscle invasive bladder cancer, 62.8% reported local side effects, including bacterial and chemical cystitis, increased frequency of urination, hematuria, and other bladder irritations. 30.6% reported systemic side effects, including fever, flu-like symptoms, lung or liver infection, rash, and sepsis, among others. The most frequent side effect was chemical cystitis (urinary tract infection induced by the chemicals rather than infection by bacteria) in 35% of patients. General malaise was the second most prevalent symptom, occurring in 15.5% of patients. 7.8% of patients were forced to stop treatment due to intolerable side effects. There was also no difference in side effects with varying dosage and treatment duration.[13]
Overall, adverse reactions to BCG immunotherapy can be confused with flu-like symptoms, with a burning sensation in the bladder being the only indication otherwise. On some rare occasions, the Bacillus Calmette-Guérin strain of Mycobacteria bovis can spread throughout the body and lead to serious infections, including sepsis or a systemic BCG reaction. This sort of systemic infection will usually occur within seven days of a traumatic catheterization, biopsy, or trans-urethral resection (bladder tumor removal) following exposure to BCG. If a fever is unresponsive to aspirin or other fever reducers, it may be an indication that a patient is experiencing some of these more serious side effects. Other symptoms of this potentially serious complication can include confusion, chills, low blood pressure and its associate symptoms such as dizziness, lightheadedness, shortness of breath, and narrowing vision. Pneumonitis, hepatitis, prostatitis, epididmal-orchitis, (characterized by swelling of the lungs, liver, prostate gland, and testes, respectively) trouble breathing and other side effects of systemic infection can be the result of Systemic BCG reaction.[1]
However, it is important to note that not everyone experiences the adverse reactions listed above, and the risk of side effects should not be a deterrent to treatment, as the positive prophylactic uses for early stage bladder cancer outweigh the risk of worsening or recurrent bladder cancer. The onset, length, and acuteness of symptoms are predictable, these symptoms improve after the course of treatment is completed, and side effects are typically easily manageable through a variety of simple symptom management strategies, such as taking pain relievers. The first 1-3 treatment sessions are usually asymptomatic, and mortality via this type of treatment is extraordinary now, given increased physician awareness and monitoring of side effects in recent years.[14] Immunotherapy remains the treatment of choice for cancers of this variety after decades of research and clinical use.
Other Uses of BCG
An alternative use for BCG is in the form of the BCG vaccine for the prevention of tuberculosis caused by infection of Mycobacterium tuberculosis. The BCG vaccine is the most-used vaccine in human history and contains live bacteria of the Calmette and Guérin strain Mycobacterium bovis. It is delivered percutaneously using a sterile multiple puncture device to persons who are have not previously been exposed to infection as determined by skin test and are at high risk of future exposure. This includes infants and children who live with tuberculosis positive family or caretakers, and health care workers in high-risk settings where patients may carry multiple antibiotic resistant M. tuberculosis strains.[15]
Efficacy of the BCG vaccine in preventing contraction of active M. tuberculosis infection is variable and controversial. In 1994, a meta-analysis based on 26 separate analyses of BCG vaccine effectiveness estimated overall efficacy of BCG vaccine in prophylactic use to be around 50%.[16] The vaccine is ineffective in treatment of active tuberculosis infection, and vaccination can result in false positive skin tests for exposure to M. tuberculosis. False positive tests for active infection with the interferon gamma release assay (IGRA) have not been reported, but should nonetheless be used together with radiography and other forms of evaluation to assess infection status.[17]
There are also potential indications for BCG immunotherapy in the treatment of colorectal cancer. Colorectal carcinoma remains one of the cancers with the highest mortality rate despite advances in cytotoxic drugs and new surgical and radiological techniques. Researchers have begun looking for therapeutic vaccines as complementary treatments to these procedures as has been done with bladder cancer for decades. The focus has been on production of tumor-associated antigens, which may be induced by therapeutic vaccination. In a trial of more than 1,300 colon cancer patients, it was found that autologous colorectal cancer cells (cells taken from the same patient), when mixed with BCG and reintroduced into the patient, stimulated a tumor-specific immune response that was of significant benefit to patients with Stage II colorectal carcinoma.[18] These results were reproducibly induced without any serious long-term side effects or creation of autoimmune disorders- a major concern of creating future immunotherapies.
Conclusions and Importance
Bacillus Calmette-Guérin Therapy is not a recent discovery, but only now are researchers beginning to fully elucidate more of the mechanism of action behind this well-established and time-tested cancer therapy. In the future, there is potential for more uses of BCG in other cancer therapies as scientists discover how this particular type of immunotherapy works and how it can be applied to other cancer types. However, there is potential for a worldwide crisis as suppliers of different BCG strains are encountering manufacturing issues and struggle to keep up with demand. To exacerbate the issue, the major BCG supplier of the Connaught strain recently announced that they will cease all production of the strain in the future.[4] The future for bladder cancer therapy is currently uncertain as these manufacturing issues pend resolution, but the outlook of the research community is hopeful.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Chemocare.com. “BCG (Bacillus Calmette-Guerin).” (n.d.).
- ↑ “Intravesical Therapy for Bladder Cancer.” 2016, January 26.
- ↑ 3.0 3.1 M. Babjuk, W. Oosterlinck, R. Sylvester, et al., “EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: the 2011 update.” 2011. Eur Urol, 59:997–1008
- ↑ 4.0 4.1 4.2 4.3 Boehm BE, et al., “Efficacy of Bacillus Calmette-Guérin Strains for the Treatment of non-muscle Invasive Bladder Cancer: a Systematic Review and Network Meta-analysis.” 2017. The Journal of Urology. doi: 10.1016/ j.juro.2017.01.086.
- ↑ CDC Fact Sheets. 2012, September 01.
- ↑ Boehm BE, et al., “Efficacy of Bacillus Calmette-Guérin Strains for the Treatment of non-muscle Invasive Bladder Cancer: a Systematic Review and Network Meta-analysis.” 2017. The Journal of Urology. doi: 10.1016/ j.juro.2017.01.086.
- ↑ 7.0 7.1 Liu, J., Tran, V., Leung, A. S., Alexander, D. C., & Zhu, B., “BCG Vaccines: Their mechanisms of attenuation and impact on safety and protective efficacy.” 2009. Human Vaccines, 5(2), 70-78. doi:10.4161/hv.5.2.7210
- ↑ Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. “The treated natural history of high risk superficial bladder cancer: 15-year outcome.” 1997. The Journal of urology. 158:62-7.
- ↑ Pagano F, Bassi P, Milani C, Meneghini A, Maruzzi D, Garbeglio A. “A low dose bacillus CalmetteGuérin regimen in superficial bladder cancer therapy: is it effective?” The Journal of urology. 1991;146:32-5.
- ↑ Böhle, A., Brandu, S., “Immune Mechanisms in Bacillus Calmette-Guerin Iummunotherapy for Superficial Bladder Cancer” The Journal of Urology. 2003. 170(3):964-969.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Redelman-Sidi et al., “The mechanism of action of BCG therapy for bladder cancer, a current perspective.” 2014. Nature Reviews Urology. 11:153-162.
- ↑ TheraCys (BCG) [package insert. Swiftwater, PA: Sanofi Pasteur; November 2015.]
- ↑ Brausi, M. et al., “Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG.” 2014. European Urology 65:1 69-76. doi:10.1016/j.eururo.2013.07.021
- ↑ Steinberg, G. D., “Bacillus Calmette-Guérin Immunotherapy for Bladder Cancer Overview of BCG Immunotherapy.” 2017, March 13.
- ↑ FDA.gov, “BCG Vaccine U.S.P. (For Precutaneous Use)”
- ↑ Colditz GA, Brewer TF, Berkeley CS et al., “Efficacy of BCG VACCINE in the prevention of tuberculosis. Meta-analysis of the published literature.” JAMA 1994. 271:698-709.
- ↑ Centers for Disease Control and Prevention. “The Role of BCG VACCINE in the Prevention and Control of Tuberculosis in the United States.” 1996. MMWR 45(RR-4):1-18
- ↑ Mosolits, S., Nilsson, B., & Mellstedt, H., “Towards therapeutic vaccines for colorectal carcinoma: a review of clinical trials.” 2005. Expert Rev Vaccines, 4(3), 329-50. doi:10.1586/14760584.4.3.329
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
