Bacillus thuringiensis toxin in C. elegans: Difference between revisions
Adrianowyczs (talk | contribs) No edit summary |
Adrianowyczs (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
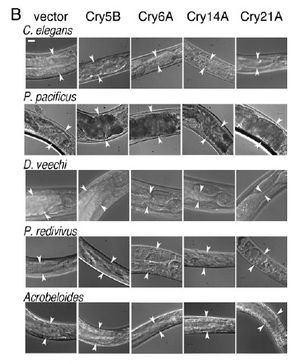
[[Image:Wei2003_1.jpeg|thumb|300px|right|(B) Photographs | [[Image:Wei2003_1.jpeg|thumb|300px|right|(B) Photographs | ||
of the anterior intestine of nematodes fed the four toxic crystal proteins in E. coli. Arrowheads delineate the width of the intestine at | of the anterior intestine of nematodes fed the four toxic crystal proteins in E. coli. Arrowheads delineate the width of the intestine at position near the | ||
anterior. (Bar 5 20 mm.)<http://www.pnas.org/content/100/5/2760.long/>]] | anterior. (Bar 5 20 mm.)<http://www.pnas.org/content/100/5/2760.long/>]] | ||
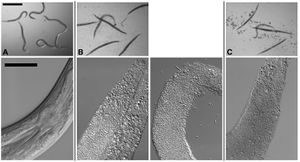
[[Image:Kho2001_1.jpg|thumb|300px|right|Figure 1. Infection of C. elegans by B. thuringiensis and B. anthracis. Top row: Dissecting microscope view of nematodes cultured under various | [[Image:Kho2001_1.jpg|thumb|300px|right|Figure 1. Infection of C. elegans by B. thuringiensis and B. anthracis. Top row: Dissecting microscope view of nematodes cultured under various | ||
conditions. Scale bar of | conditions. Scale bar of images in top row is 500 mm. Bottom row: Compound microscope view of nematodes cultured under various conditions. | ||
For all images in the bottom row, anterior of the worm is top right and scale bar is 50 mm. (A) C. elegans cultured in a well with B. thuringiensis without | For all images in the bottom row, anterior of the worm is top right and scale bar is 50 mm. (A) C. elegans cultured in a well with B. thuringiensis without Cry5B. Top row: None of the six nematodes are infected. All are healthy. The blur associated with some of the worms in the top row is due to their movement in the well. Bottom row: The internal structures of C. elegans fed B. thuringiensis without Cry5B, including the pharynx and intestine, are all intact. (B) C. elegans cultured in a well with B. thuringiensis and Cry5B. Top row: Five of the six worms are completely infected (rigid, lack of internal | ||
Cry5B. Top row: None of the six nematodes are infected. All are healthy. The blur associated with some of the worms in the top row is due to their | structures and normal coloration); one is not. Bottom row: Infected animals show complete or near complete digestion of internal structures by the bacteria. Vegetative and sporulated bacteria can be seen in these lethally infected animals. (C) Similar images as in (B) except the bacterium cultured with the nematodes is Bacillus anthracis. <http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0029122/>]] | ||
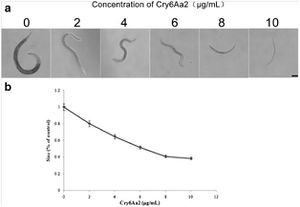
movement in the well. Bottom row: The internal structures of C. elegans fed B. thuringiensis without Cry5B, including the pharynx and intestine, are all | [[Image:Luo2013_1.jpg|thumb|300px|right|Growth assay of L1 larvae of C. elegans with Cry6Aa2 toxin. a Microscope views of worms cultured in gradient doses of Cry6Aa2 toxin. Scale bar is 100 μm. b The size percentages of worms cultured in a serial dose of Cry6Aa2 toxin to worms cultured in the absence of Cry6Aa2 toxin. Data represent the average of 20 measurements for each toxin concentration. | ||
intact. (B) C. elegans cultured in a well with B. thuringiensis and Cry5B. Top row: Five of the six worms are completely infected (rigid, lack of internal | Error bars denote standard deviation <http://link.springer.com/article/10.1007%2Fs00253-013-5249-3/fulltext.html/>]] | ||
structures and normal coloration); one is not. Bottom row: Infected animals show complete or near complete digestion of internal structures by the | |||
bacteria. Vegetative and sporulated bacteria can be seen in these lethally infected animals. (C) Similar images as in (B) except the bacterium cultured | |||
with the nematodes is Bacillus anthracis. <http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0029122/>]] | |||
[[Image:Luo2013_1.jpg|thumb|300px|right|Growth assay of L1 larvae | |||
of C. elegans with Cry6Aa2 | |||
toxin. a Microscope views of | |||
worms cultured in gradient doses | |||
of Cry6Aa2 toxin. Scale bar is | |||
100 μm. b The size percentages | |||
of worms cultured in a serial dose | |||
of Cry6Aa2 toxin to worms | |||
cultured in the absence of | |||
Cry6Aa2 toxin. Data represent | |||
the average of 20 measurements | |||
for each toxin concentration. | |||
Error bars denote standard | |||
deviation <http://link.springer.com/article/10.1007%2Fs00253-013-5249-3/fulltext.html/>]] | |||
<br>By Sarah Adrianowycz<br> | <br>By Sarah Adrianowycz<br> | ||
== | ==Bacillus thuringiensis as an organism== | ||
<br> | <br> Bacillus thuringiensis is prevalent in the soil, and is a gram –positive, spore-forming member of the Bacillus ceres group. The Bacillus ceres group is a collection of seven genetically similar species, three of which are so alike that they are occasionally considered the same organism, except for their vastly different pathogenic outcomes (Kho et al. 2011). It is these different pathogens, or external expression of the organism’s phenotype, which are often used by researchers to determine which species are distinct. In the case of B. thuringiensis the organism is typified by the production of specific invertebrate related pathogens (Ceuppens et al., 2013). However, the issue with using phenotypic differences to differentiate among these organisms is that the differences frequently stem from virulence factors contained on plasmids, which are extremely variable and transient. These genetic elements separate from the primary genome can be exchanged through horizontal gene transmission, lost in the environment, or selected against during culturing in the laboratory. Despite the issues with distinguishing strains based upon their ability to cause disease, B. thuringiensis is often distinguished by the ability to form crystal proteins. These products are insecticidal in nature and referred to as cry genes for the genes that code their expression (Ceuppens et al.). In the wild, most strains of B. thuringiensis are capable of producing anywhere from 1-4 types of cry proteins (Kho et al., 2011).The ability to produce multiple cry genes may be explained because of the synergistic effect that some of these proteins have in taking over a host when working together, or because different potential hosts have different levels of susceptibility to specific cry proteins (E. Schnephf, 1998).<br> | ||
<br>It is the ability to produce these crystal proteins that gives B. thuringiensis value as an insecticide and has made it especially interesting to humans who are looking into biological applications of the cry proteins. These toxins have been utilized both in direct application on to crops and as a source of transgenic elements that provide resistance against the orders Lepidoptera, Diptera, and Coleoptera (E. Schnepf, 1998) when incorporated into the genomes of genetically modified organisms. The method of action within this subset of proteins makes them especially effective against insects but hasn’t produced evidence of negative effects against either humans or other higher trophic level organisms, as is typically the case for insecticides. Because of these perceived benefits and the lack of obvious repercussions, the utilization of plants with cry proteins has become common place since 1996, when genetically modified potatoes, corn, and potatoes with versions of cry genes were made commercially available (Schnepf). | |||
Revision as of 01:48, 23 April 2014
By Sarah Adrianowycz
Bacillus thuringiensis as an organism
Bacillus thuringiensis is prevalent in the soil, and is a gram –positive, spore-forming member of the Bacillus ceres group. The Bacillus ceres group is a collection of seven genetically similar species, three of which are so alike that they are occasionally considered the same organism, except for their vastly different pathogenic outcomes (Kho et al. 2011). It is these different pathogens, or external expression of the organism’s phenotype, which are often used by researchers to determine which species are distinct. In the case of B. thuringiensis the organism is typified by the production of specific invertebrate related pathogens (Ceuppens et al., 2013). However, the issue with using phenotypic differences to differentiate among these organisms is that the differences frequently stem from virulence factors contained on plasmids, which are extremely variable and transient. These genetic elements separate from the primary genome can be exchanged through horizontal gene transmission, lost in the environment, or selected against during culturing in the laboratory. Despite the issues with distinguishing strains based upon their ability to cause disease, B. thuringiensis is often distinguished by the ability to form crystal proteins. These products are insecticidal in nature and referred to as cry genes for the genes that code their expression (Ceuppens et al.). In the wild, most strains of B. thuringiensis are capable of producing anywhere from 1-4 types of cry proteins (Kho et al., 2011).The ability to produce multiple cry genes may be explained because of the synergistic effect that some of these proteins have in taking over a host when working together, or because different potential hosts have different levels of susceptibility to specific cry proteins (E. Schnephf, 1998).
It is the ability to produce these crystal proteins that gives B. thuringiensis value as an insecticide and has made it especially interesting to humans who are looking into biological applications of the cry proteins. These toxins have been utilized both in direct application on to crops and as a source of transgenic elements that provide resistance against the orders Lepidoptera, Diptera, and Coleoptera (E. Schnepf, 1998) when incorporated into the genomes of genetically modified organisms. The method of action within this subset of proteins makes them especially effective against insects but hasn’t produced evidence of negative effects against either humans or other higher trophic level organisms, as is typically the case for insecticides. Because of these perceived benefits and the lack of obvious repercussions, the utilization of plants with cry proteins has become common place since 1996, when genetically modified potatoes, corn, and potatoes with versions of cry genes were made commercially available (Schnepf).
