Batrachochytrium salamandrivorans
By Fiona Ellsworth
Introduction
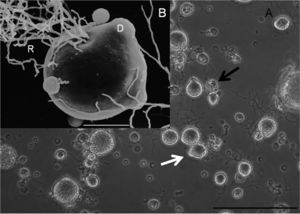
]. A thallus is a plant body without stem, roots, or other appendages, and are typical of fungi[8]. A monocentric thallus produces a single reproductive organ, a zoospore in the case of Bsal. Occasionally a colonial thallus is seen, which is a thallus that produces more than a single reproductive organ. Zoospores are generally spherical in shape, with a diameter on average 4.6 um, an uneven surface dotted with protrusions, and a single flagellum for facilitation of movement. Sporangium form (on average with a diameter of 27.9 um) at the tip of discharge tubes as the mechanism to release zoospores. Bd does not form these tubes in vitro, and neither does it produce as many colonial thalli either in vitro or in vivo. Another morphological difference between these two strains is their thermal tolerance. Bsal has a distinctly lower thermal tolerance than Bd, with its optimal temperature range for growth between 10℃ and 15℃[4]. Bd has been known to grow best at temperatures between 17℃ and 25℃, a range which shows a distinct lack of overlap with Bsal’s optimal temperature range. Furthermore, Bsal has been known to grow at temperatures as low as 5℃, and dies at temperatures greater than 25℃. While these are fairly narrow temperature ranges for optimal growth for both Bd and Bsal, it is suspected that both of these species would exhibit temperature adaptations to the environments in which they find themselves, therefore these temperatures ranges might not be absolute. This is worrisome for efforts to limit the spread and impact of these fungal pathogens on worldwide amphibian populations.
Skin scrapes of infected salamanders with Bsal illustrate the effects of this fungus on its host[1]. Colonial thalli are seen throughout all epidermal layers, and cause skin lesions and deep ulcerations on the infected organism. Around the skin erosions were damaged keratinocytes, keratin producing epidermal cells, each containing a Bsal thallus. Many of these epidermal cells showed signs of hyperplasia (abnormal enlargement) and hyperkeratosis (thickening of the outer layer of skin). No signs of Bd were found in any of the Bsal infected organisms, further exemplifying each of the strains’ very specific niches. Individuals infected with Bsal experienced death around 7 days after infection, after going through a series of health problems including anorexia, apathy, and ataxia (the loss of control over body movement). Not only has Bsal been experimentally shown to be extremely deadly to its amphibian host, but it is also highly infectious. Housing infected and uninfected individuals together has demonstrated infection rates of previously uninfected individuals of around 25 days. Furthermore, deceased infected individuals retain the virus for a brief period after death, increasing the possibility of transmission. Death soon follows infection.
Laboratory Detection of Bsal
Effective methods of detection of Bsal included skin scrapes and subsequent PCR[1]. Martel designed species-specific PCR primers for the purpose of easily identifying Bsal infected amphibians, a much needed tool for combating the spread of the pathogen. These primers, STerF and STerR, amplify the 5.8S rRNA gene and the adjacent transcribed spacer regions, ITS1 and ITS2. With these primers, Martel et al was experimentally able to identify the presence of Bsal in all tested amphibian tissues, even several samples from already deceased individuals. Perhaps most importantly, this PCR method did not produce results from Bd infected individuals, thereby offering researchers an effective way to test for Bsal alone and not Bd in amphibian populations. This reliable specificity is highly important to the containment and discouragement of Bsal.
Transmission: Pathogen Host Interactions
Stegen et al (2015) found that one of the factors that contribute to the extreme vulnerability of salamander populations to Bsal is the fast rate of host population collapse, once Bsal has been introduced[7]. This result is largely due to the high susceptibility of adults to the fungal pathogen, whose mass die offs then bring about a shift in the demographics of the population, which inhibits recovery. Adults are more susceptible than juveniles because of their increased interaction with potential pathogen hosts, through sexual intercourse, territorial disputes, or trips back and forth to where eggs are laid. Because Bsal is highly infectious through physical contact, this places adults at a much higher risk of infection than juveniles. Stegen et al further found that the effect of infection on a host is dependent on the concentration of the pathogen and the temperature. Furthermore, there appeared to be no immune defense mounted against the pathogen, leading to rapid succumbing of the host. Through experimentation, Stegen et al found that this lack of an immune response did not change depending on the concentration of the pathogen. Even at low concentrations, host were unable to erect any sort of defense, thereby eliminating vaccination as a potential method of pathogen elimination in populations. The only difference that low concentrations of pathogens seemed to cause was a slower buildup of infection. Ultimately, however, the infected individual died.
Treatment
The established antimycotic (antifungal) treatment for Bd has failed in its use against Bsal. Blooi et al, in a series of experiments performed on fire salamanders from populations in the Netherlands, suggest that this is due to different minimum inhibitory concentrations (MIC) necessary for use against Bd versus Bsal[9]. Furthermore, use of antimycotic agents in conjunction with one another, as opposed to separately, was documented to cure infected salamanders of Bsal. Blooi et al also established the ability of thermal treatments in eliminating Bsal from its host. However, the temperature range at which Bsal begins to die is also at the temperature limit for which most salamander species can survive, rendering thermal treatments useless in widespread combatting of the fungal pathogen. Blooi et al tested MIC levels for several antimycotic agents commonly used against Bd, including Florfenicol, Voriconazole, Polymyxin E, Itraconazole, and Terbinafine. They found some of the MIC levels to be higher for Bsal, and some to be lower, when compared to Bd. This explains the widespread failure of these agents, at the levels used for Bd, to effectively treat Bsal. They also found that while these treatments on their own and at the proper MICs, could inhibit Bsal growth, they could rarely eliminate the fungal pathogen. However, Polymyxin E used alongside Itraconazole or Voriconazole could actually clear the salamander host of infection. Furthermore, temperature in conjunction with these antimycotic agents played a key role in clearing of the pathogen from the host. Blooi et al found that raising the temperature to 20℃ substantially improved the efficacy of the antimycotic treatments, and was particularly useful for salamander species that could not survive temperatures greater than 25℃, the limit for Bsal survivorship [6].
Etymology
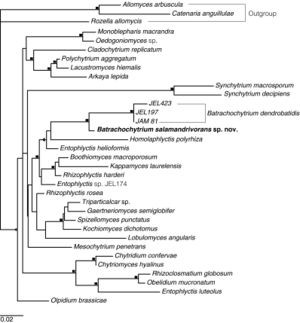
Batrachochytrium salamandrivorans and Batrachochytrium dendrobatidis are the two members in a clade of Chytridiomycota, characterized as parasites that infect amphibian hosts with a mostly lethal outcome. Martel et al (2014) used Bayesian estimates of divergence time to suggest that Bsal diverged from Bd 67.3 million years ago, in the late Cretaceous or early Paleogene[3]. For much of this time, Bsal was confined to Asia, in several reservoir species likely including Cynops pyrrhogaster, Cynops cyanurus, and Paramesotriton deloustali. These three species are unfortunately actively involved in the international pet trade[5]. Martel et al estimate that the potential for being a reservoir evolved in the ancestors of these modern Asian newts between 55 to 34 million years ago, in the Paleogene[3]. While the timeline for the arrival of Bsal in Asia and the evolution of resistance are relatively close on a scale of millions of years, there is still the difference of multiple millions of years between arrival and resistance, a length of time currently unavailable to amphibians in the rapidly changing environment of today’s planet.
Efforts to Address the Rise of Bsal
In January 2017, the US passed the Lacey Act which attempts to restrict listed “injurious” species from transport into and within the country[10]. Several salamander genera were included on the injurious species list, due to their likelihood of spreading Bd and Bsal to other populations. The Lacey Act however was curtailed in April 2017 when the Supreme Court ruled that it only applied to cross-state transport, not transport of injurious species within states. This interpretation will likely limit the ability of the Lacey Act to prevent against the spread of Bsal[6].
Because of the length of time for which Bd has been known to the scientific community, more has been observed and written about Bd than Bsal. However, scientists are making an active effort to learn more about these fungal pathogens and their interactions with their host in an effort to mitigate the already extreme losses of the sixth mass extinction[1][3][6]. The more that is known, the better able conservationists will be able to protect these amphibian species. As has been mentioned here, An Martel has been at the forefront of identification and observation of Bsal[1][3].
Yap et al used salamander pet trade data, salamander hotspots, and habitat suitability for reservoir species to map projected regions of North America at high risk of a Bsal outbreak[11]. They concluded that the North Western coast (along Washington State and leading into Canada), Northern Florida and the rest of the American South East, and Mexico City (likely because of the prevalence of the pet trade, particularly coming from Asia, here) were at the highest risk of an outbreak of the fungal pathogen.
After assessing the high risk North America, an invaluable hotspot for salamander biodiversity and species richness, faces from a Bsal epidemic, Yap et al advocates for preventative measures as opposed to reactive ones. Preventative actions would be more cost effective and have a higher likelihood of success. Yap et al specifically recommends that the US halt all pet trading activity. While this seems extreme, the potential for the arrival of Bsal in the Americas is incredibly high, and once here, the consequences are dire. This is further exacerbated by the fact that as of yet, there is no entirely successful method known to combat the spread of Bsal once it has gained a footing in a population. Until a cure or other form of management is found, prevention is likely the best mode of action. Yap et al note that there is little internationally agreed upon mandatory regulation on EID’s (emerging infectious diseases), particularly animal ones. Some such framework of international cooperation to detect and quarantine infected individuals is necessary in this current climate of frequent global travel and exchange.
References
- ↑ Martel, An et al. “Batrachochytrium Salamandrivorans Sp. Nov. Causes Lethal Chytridiomycosis in Amphibians.” Proceedings of the National Academy of Sciences of the United States of America 110.38 (2013): 15325–15329. PMC.
- ↑ Longcore, Joyce E., et al. “Batrachochytrium Dendrobatidis Gen. Et Sp. Nov., a Chytrid Pathogenic to Amphibians.” Mycologia, vol. 91, no. 2, 1999, pp. 219–227.
- ↑ Martel et al. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 31 Oct 2014: Vol. 346, Issue 6209, pp. 630-631.
- ↑ Stevenson, L. et al. “Variation in Thermal Performance of a Widespread Pathogen, the Amphibian Chytrid Fungus Batrachochytrium Dendrobatidis .” Ed. Brian Gratwicke. PLoS ONE 8.9 (2013): e73830. PMC.
- ↑ Yap, Tiffany & Koo Michelle. Batrachochytrium salamandrivorans: Deadly fungal threat to salamanders. Amphibiaweb. 31 July 2015.
- ↑ Klocke et al B. Batrachochytrium salamandrivorans not detected in U.S. survey of pet salamanders. Scientific Reports vol 7, Article number: 13132 (2017)
- ↑ Stegen et al. Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature volume 544, pages 353–356.
- ↑ [Slonczewski, J. L., & Foster, J. W. (2017). Microbiology: An Evolving Science: Third International Student Edition. WW Norton & Company.]
- ↑ Blooi et al. Successful treatment of Batrachochytrium salamandrivorans infections in salamanders requires synergy between voriconazole, polymyxin E and temperature. Scientific Reports vol 5, Article number: 11788 (2015)
- ↑ The Lacey Act. US Fish and Wildlife Service: International Affairs Website.
- ↑ Yapp et al. Averting a North American biodiversity crisis. Science 31 Jul 2015: Vol. 349, Issue 6247, pp. 481-482.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.