Bifidobacterium animalis: Difference between revisions
| (62 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
==Classification== | ==Classification== | ||
[[File:bifidobacteriumanimalis.jpg]] | |||
Scanning electron micrograph (SEM) of ''Bifidobacterium animalis'' | |||
==='''Higher Order Classification'''=== | ==='''Higher Order Classification'''=== | ||
Domain: ''Bacteria'' | Domain: ''Bacteria'' | ||
| Line 20: | Line 25: | ||
==Description and Significance== | ==Description and Significance== | ||
''Bifidobacterium animalis'' is a branched, rod-shaped (diplobicillus) Gram-positive bacteria. This bacteria is generally found in the large intestines of mammals such as: cats, cows, and humans. | |||
Discovered by Henry Tissier in 1900 | Discovered by Henry Tissier in 1900, ''Bifidobacterium animalis'' was isolated from a breast-fed infant[1]. | ||
Although it was discovered in 1900, research on this bacterium did not ensue until the interest in probiotics in 1950. Probiotics are microorganisms that are ingested either in combination or as a single organism in an effort to normalize intestinal microbiota and potentially improve intestinal barrier function[ | Although it was discovered in 1900, research on this bacterium did not ensue until the interest in probiotics in 1950. Probiotics are microorganisms that are ingested either in combination or as a single organism in an effort to normalize intestinal microbiota and potentially improve intestinal barrier function[2]. It has long been speculation that probiotics are useful in alleviating or curing the symptoms associated with irritable bowel syndrome, ulcerative colitis, diarrhea, and atopic dermatitis[3]. It is also suggested that ''Bifidobacterium animalis'' can reduce fat content and prevent obesity. In a probiotic experiment on ''C. elegans'' it was determined that ''Bifidobacterium animalis'' was the most effective in showing fat reduction[9]. There is even evidence that it speeds up the colonic transit time in healthy humans[10][11]. | ||
==Genome Structure== | ==Genome Structure== | ||
''Bifidobacterium animalis'' contains one circular chromosome consisting of approximately 1, 943, 990 base pairs with a G-C content of 60.5% which is similar to all members of ''Bifidobacterium''[4]. The bacterium carries 1,560 open reading frames (OFRs), 52 tRNA operons, 4 rRNA operons, and a 36 base pair cluster of regularly interspersed short palindromic repeat (CRISPR)[4]. | |||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
''Bifidobacterium animalis'' contains all of the traditional elements of rod-shaped bacteria including organelles, a plasmid, a cell membrane, and cell wall but not a flagella. Recent studies of the extracellular polysaccharides of the cell envelope have found high RHa residue and terminal Gal furanoside, which has been postulated to interact with the mucous community of the intestines[3]. | |||
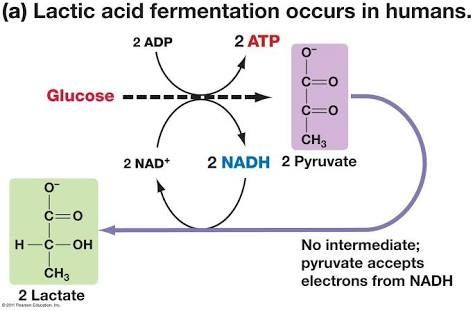
''Bifidobacterium animalis'' is an anaerobic chemoorganotroph that undergoes lactic acid fermentation. Lactic acid fermentation is the process by which six-carbon sugars like glucose are converted into cellular energy and lactate in the absence of oxygen[5]. Lactic acid in the intestines is hypothesized to aid in the hydrolysis of lactose which is not easily broken down[6]. "''Bifidobacteria'' can utilize a diverse range of dietary carbohydrates that escape degradation in the upper parts of the intestine, many of which are plant-derived oligo- and polysaccharides" [7]. | |||
[[File:main-qimg-2d62a355a4c1fc399a411a1093c69f44-c.jpg]] | |||
Like most prokaryotic bacteria, ''Bifidobacterium animalis'' reproduces and continues its life cycle through binary fission which is a form of asexual reproduction that involves replicating the cell's genetic material followed by the parent cell dividing into two equal sized clone daughter cells[8]. | |||
[https://youtu.be/_FBBnNhN_NM "binary fission video"] | |||
==Ecology== | ==Ecology== | ||
''Bifidobacterium animalis'' has an ecological habitat that helps make up the colon flora of most mammals. It resides mainly in the large intestine in a pH from 5.5-7. This bacterium is commonly cultured in a [https://www.sigmaaldrich.com/catalog/product/sial/69966?lang=en®ion=US "MRS broth"] at 37 degrees Celsius[3]. | |||
Much research has gone into deciphering what element plays into the symbiotic relationship that probiotics like ''Bifidobacterium animalis'' form with the gastrointestinal tract of most mammals. The formation of lactic acid allows for the hydrolysis of lactose and other complex sugars, and it is believed that sugars present on the membrane of the bacterium are capable of interacting with the mucous lining of the large intestines. This structure of sugars along the membrane are believed to contribute to the bacteria's ability to regulate biological and physiological functionalities in the host gastrointestinal tract[3]. There is research done on the pathology of the intestines suggesting that ''Bifidobacterium animalis'' protects intestinal cells from the inflammation-associated response caused by ''E. coli'' by partly reducing pathogen adhesion and by counteracting neutrophil migration, probably through the regulation of chemokine and cytokine expression[12]. | |||
==References== | ==References== | ||
[ | [1]Tissier, H. Recherches sur la flore intestinale des nourrissons:(état normal et pathologique) (Doctoral dissertation). 1900. | ||
[2] Ghouri, Yezaz A et al.[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4266241/ "Systematic Review of Randomized Controlled Trials of Probiotics, Prebiotics, and Synbiotics in Inflammatory Bowel Disease."], Clinical and Experimental Gastroenterology 7. 2014. pp 473–487. PMC. Web. 18 April 2018. | |||
[3] Uemura, Yusuke et al. [https://www.ncbi.nlm.nih.gov/pubmed/25043203 "Chemical structure of the cell wall-associated polysaccharide of ''Bifidobacterium animalis'' subsp. ''lactis'' LKM512"], Glycoconjugate Journal. November 2014, vol 31, issue 8, pp 555–561. Web. 18 April. 2018. | |||
[4] Bottacini, Francesca et al. [http://jb.asm.org/content/193/22/6387.short "Complete Genome Sequence of ''Bifidobacterium animalis'' subsp. ''lactis'' BLC1"], Journal of Bacteriology. November 2011. vol 193, issue 22, pp 6387-6388. Web. 18 April. 2018. | |||
[5] Robert Bear, David Rintoul, Bruce Snyder, Martha Smith-Caldas, Christopher Herren, Eva Horne, Principles of Biology. OpenStax CNX. May 13, 2016 http://cnx.org/contents/db89c8f8-a27c-4685-ad2a-19d11a2a7e2e@24.18. | |||
[6] Gilliland, Stanley. [https://www.sciencedirect.com/science/article/pii/037810979090705U "Health and nutritional benefits from lactic acid bacteria"], ScienceDirect. September 1990. vol 87, issue 1–2, pp 175-188. Web. 18 April 2018. | |||
[7] Pokusaeva, K., Fitzgerald, G. F., & van Sinderen, D. [http://doi.org/10.1007/s12263-010-0206-6 "Carbohydrate metabolism in Bifidobacteria"], Genes & Nutrition. February 2011. vol 6, issue 3, pp 285–306. Web. 18 April 2018. | |||
[8] Otieno, Daniel. [https://link.springer.com/chapter/10.1007%2F978-3-642-20838-6_1 "Biology of Prokaryotic Probiotics"], Springer Link. June 2011. vol 21, pp 1-28. Web. 18 April 2018. | |||
[9] Aleixandre, Amaya. [https://pubs.acs.org/doi/abs/10.1021/acs.jafc.5b05934 "Probiotic Strain ''Bifidobacterium animalis'' subsp. ''lactis'' CECT 8145 Reduces Fat Content and Modulates Lipid Metabolism and Antioxidant Response in ''Caenorhabditis elegans''], J. Agric. Food Chem. April 2016. vol 64, issue 17, pp 3462–3472. Web. 18 April 2018. | |||
[10] Marteau, P. [https://onlinelibrary.wiley.com/doi/full/10.1046/j.1365-2036.2002.01188.x "Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study"], Alimentary Pharmacology & Therapeutics. March 2002. vol 16, issue 3, pp 587-593. Web. 18 April 2018. | |||
[11] Bouvier, M. [https://www.jstage.jst.go.jp/article/bifidus1996/20/2/20_2_43/_article/-char/ja/ "Effects of Consumption of a Milk Fermented by the Probiotic Strain ''Bifidobacterium animalis'' DN-173 010 on Colonic Transit Times in Healthy Humans"], J-Stage. 2001-2002. vol 20, issue 2, pp 43-48. Web. 18 April 2018. | |||
[ | [12] Roselli, M., Finamore, A., Britti, M., & Mengheri, E. [https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/probiotic-bacteria-bifidobacterium-animalis-mb5-and-lactobacillus-rhamnosus-gg-protect-intestinal-caco-2-cells-from-the-inflammation-associated-response-induced-by-enterotoxigenic-escherichia-coli-k88/CC3C5694FDF3F4F95EEE91D1EE1A8243 "Probiotic bacteria ''Bifidobacterium animalis'' MB5 and ''Lactobacillus rhamnosus'' GG protect intestinal Caco-2 cells from the inflammation-associated response induced by enterotoxigenic ''Escherichia coli'' K88"], British Journal of Nutrition. June 2006. vol 95, issue 6, pp 1177-1184. Web. 18 April 2018. | ||
==Author== | ==Author== | ||
Latest revision as of 19:05, 18 April 2018
Classification
Scanning electron micrograph (SEM) of Bifidobacterium animalis
Higher Order Classification
Domain: Bacteria
Phylum: Actinobacteria
Class: Actinobacteria
Order: Bifidobacteriales
Family: Bifidobacteriaceae
Species
Bifidobacterium animalis
Description and Significance
Bifidobacterium animalis is a branched, rod-shaped (diplobicillus) Gram-positive bacteria. This bacteria is generally found in the large intestines of mammals such as: cats, cows, and humans. Discovered by Henry Tissier in 1900, Bifidobacterium animalis was isolated from a breast-fed infant[1].
Although it was discovered in 1900, research on this bacterium did not ensue until the interest in probiotics in 1950. Probiotics are microorganisms that are ingested either in combination or as a single organism in an effort to normalize intestinal microbiota and potentially improve intestinal barrier function[2]. It has long been speculation that probiotics are useful in alleviating or curing the symptoms associated with irritable bowel syndrome, ulcerative colitis, diarrhea, and atopic dermatitis[3]. It is also suggested that Bifidobacterium animalis can reduce fat content and prevent obesity. In a probiotic experiment on C. elegans it was determined that Bifidobacterium animalis was the most effective in showing fat reduction[9]. There is even evidence that it speeds up the colonic transit time in healthy humans[10][11].
Genome Structure
Bifidobacterium animalis contains one circular chromosome consisting of approximately 1, 943, 990 base pairs with a G-C content of 60.5% which is similar to all members of Bifidobacterium[4]. The bacterium carries 1,560 open reading frames (OFRs), 52 tRNA operons, 4 rRNA operons, and a 36 base pair cluster of regularly interspersed short palindromic repeat (CRISPR)[4].
Cell Structure, Metabolism and Life Cycle
Bifidobacterium animalis contains all of the traditional elements of rod-shaped bacteria including organelles, a plasmid, a cell membrane, and cell wall but not a flagella. Recent studies of the extracellular polysaccharides of the cell envelope have found high RHa residue and terminal Gal furanoside, which has been postulated to interact with the mucous community of the intestines[3].
Bifidobacterium animalis is an anaerobic chemoorganotroph that undergoes lactic acid fermentation. Lactic acid fermentation is the process by which six-carbon sugars like glucose are converted into cellular energy and lactate in the absence of oxygen[5]. Lactic acid in the intestines is hypothesized to aid in the hydrolysis of lactose which is not easily broken down[6]. "Bifidobacteria can utilize a diverse range of dietary carbohydrates that escape degradation in the upper parts of the intestine, many of which are plant-derived oligo- and polysaccharides" [7].
Like most prokaryotic bacteria, Bifidobacterium animalis reproduces and continues its life cycle through binary fission which is a form of asexual reproduction that involves replicating the cell's genetic material followed by the parent cell dividing into two equal sized clone daughter cells[8]. "binary fission video"
Ecology
Bifidobacterium animalis has an ecological habitat that helps make up the colon flora of most mammals. It resides mainly in the large intestine in a pH from 5.5-7. This bacterium is commonly cultured in a "MRS broth" at 37 degrees Celsius[3].
Much research has gone into deciphering what element plays into the symbiotic relationship that probiotics like Bifidobacterium animalis form with the gastrointestinal tract of most mammals. The formation of lactic acid allows for the hydrolysis of lactose and other complex sugars, and it is believed that sugars present on the membrane of the bacterium are capable of interacting with the mucous lining of the large intestines. This structure of sugars along the membrane are believed to contribute to the bacteria's ability to regulate biological and physiological functionalities in the host gastrointestinal tract[3]. There is research done on the pathology of the intestines suggesting that Bifidobacterium animalis protects intestinal cells from the inflammation-associated response caused by E. coli by partly reducing pathogen adhesion and by counteracting neutrophil migration, probably through the regulation of chemokine and cytokine expression[12].
References
[1]Tissier, H. Recherches sur la flore intestinale des nourrissons:(état normal et pathologique) (Doctoral dissertation). 1900.
[2] Ghouri, Yezaz A et al."Systematic Review of Randomized Controlled Trials of Probiotics, Prebiotics, and Synbiotics in Inflammatory Bowel Disease.", Clinical and Experimental Gastroenterology 7. 2014. pp 473–487. PMC. Web. 18 April 2018.
[3] Uemura, Yusuke et al. "Chemical structure of the cell wall-associated polysaccharide of Bifidobacterium animalis subsp. lactis LKM512", Glycoconjugate Journal. November 2014, vol 31, issue 8, pp 555–561. Web. 18 April. 2018.
[4] Bottacini, Francesca et al. "Complete Genome Sequence of Bifidobacterium animalis subsp. lactis BLC1", Journal of Bacteriology. November 2011. vol 193, issue 22, pp 6387-6388. Web. 18 April. 2018.
[5] Robert Bear, David Rintoul, Bruce Snyder, Martha Smith-Caldas, Christopher Herren, Eva Horne, Principles of Biology. OpenStax CNX. May 13, 2016 http://cnx.org/contents/db89c8f8-a27c-4685-ad2a-19d11a2a7e2e@24.18.
[6] Gilliland, Stanley. "Health and nutritional benefits from lactic acid bacteria", ScienceDirect. September 1990. vol 87, issue 1–2, pp 175-188. Web. 18 April 2018.
[7] Pokusaeva, K., Fitzgerald, G. F., & van Sinderen, D. "Carbohydrate metabolism in Bifidobacteria", Genes & Nutrition. February 2011. vol 6, issue 3, pp 285–306. Web. 18 April 2018.
[8] Otieno, Daniel. "Biology of Prokaryotic Probiotics", Springer Link. June 2011. vol 21, pp 1-28. Web. 18 April 2018.
[9] Aleixandre, Amaya. "Probiotic Strain Bifidobacterium animalis subsp. lactis CECT 8145 Reduces Fat Content and Modulates Lipid Metabolism and Antioxidant Response in Caenorhabditis elegans, J. Agric. Food Chem. April 2016. vol 64, issue 17, pp 3462–3472. Web. 18 April 2018.
[10] Marteau, P. "Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study", Alimentary Pharmacology & Therapeutics. March 2002. vol 16, issue 3, pp 587-593. Web. 18 April 2018.
[11] Bouvier, M. "Effects of Consumption of a Milk Fermented by the Probiotic Strain Bifidobacterium animalis DN-173 010 on Colonic Transit Times in Healthy Humans", J-Stage. 2001-2002. vol 20, issue 2, pp 43-48. Web. 18 April 2018.
[12] Roselli, M., Finamore, A., Britti, M., & Mengheri, E. "Probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal Caco-2 cells from the inflammation-associated response induced by enterotoxigenic Escherichia coli K88", British Journal of Nutrition. June 2006. vol 95, issue 6, pp 1177-1184. Web. 18 April 2018.
Author
Page authored by Paul Proctor & Benjamin McKinnon-Duggins, student of Prof. Jay Lennon at IndianaUniversity.