Blautia Hydrogenotrophica
Classification
Bacteria; Firmicutes; Clostridia; Clostridiales; Blautia
Species
Blautia hydrogenotrophica
|
NCBI: Taxonomy |
Description and Significance
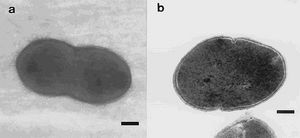
Blautia hydrogenotrophica, formerly known as Ruminococcus hydrogenotrophicus, is a species identified within the guts of mammals (humans and ruminants). B. hydrogenotrophica is a non-motile coccobacillus, 0.7-0.6 micrometers in size, occurs singly or in pairs (Bernalier et al., 1996) (Figure 1). The bacterium is strictly anaerobic; it metabolizes H2/CO2 to acetate, ferments cellobiose, fructose, galactose, glucose, lactose, maltose, mannose, raffinose, salicin, trehalose, pyruvate, formate, and esculin. The optimum habitat of Blautia hydrogenotrophica has a pH range of 6.0-7.0, and temperature of 35-37˚C. Cultured growth does not require the presence of yeast extract, tryptone, and rumen fluid (Bernalier et al., 1996). H2/CO2 acetogenesis is of great interest for human nutrition and health by decreasing the total gas volume in the colon and by producing a non-gaseous metabolite that is an energy source for eukaryotic cells (Bernalier et al., 1996). Blautia hydrogenotrophica may also be a significant microbe in the replacement of methanogens within ruminant livestock to limit the amount of methane released (Williams et al. 2009).
Genome Structure
Blautia Hydrogenotrophica strain DSM 10507 contains 3,565,428 base pairs (NCBI, 2011). The G+C content of the DNA is 45.2 mol% (Bernalier et al. 1996). Through in silico analysis of the Blautia hydrogenotrophica genome, it was found that all of the genes involved in the Wood-Ljungdahl pathway were present. This includes eight genes that are thought to encode subunits of iron-only hydrogenases. This pathway is used to convert glucose to acetate when H2 and CO2 are the only energy and carbon sources. (Bain et al., 2010)
Cell Structure, Metabolism and Life Cycle
The Blautia hydrogenotrophica is gram-positive, non-sporulating, coccobacillus shaped bacterium with an average size of 0.7-0.6 mm. Through the use of negatively stained cells, it can be observed that the organism lacks flagella (Bernalier et al. 1996). Hydrogenotrophica, meaning feeds on hydrogen, refers to the organism’s ability to use H2 and CO2 as energy source for growth” (Liu et al., 2008). Blautia hydrogenotrophica grows autotrophically by metabolizing H2 and CO2 to form acetate as sole metabolite. The organism is also able to grow heterotrophically using several different organic compounds as a substrate. Through the fermentation of glucose and fructose, acetate is the primary product, however ethanol, lactate and, to a lesser extent, isobutyrate and isovalerate can also be formed. (Bernalier et al. 1996)
The production of acetate from H2 and CO2 occurs through the acetyl-CoA pathway. Acetogens, such as Blautia hydrogenotrophica, have the ability to use a broad range of substrates, including mono-, di-, tri-, and tetrasaccharides, in addition to aromatic and aliphatic amino acids. By targeting aliphatic and aromatic amino acids, the efficiency of fermentation is increased. This is accomplished through the consumption of reducing equivalents, thus a high NAD+/NADH ratio is maintained and acetate production increases. Due to the variety of substrates that can be used, it is hypothesized that acetogens possess an advantage in fitness when competing for H2 and formate over methanogens and sulfate reducing bacteria, other groups of microorganisms found in the gut. Further support of this hypothesis comes from studies indicating acetogens’ ability to grow mixotrophically, meaning that organic substrates and H2 are used as an energy source simultaneously. This indicates that Blautia hydrogenotrophica would have a greater advantage over H2-consuming methanogens. (Bain et al., 2010)
Ecology
Blautia hydrogenotrophica inhabits the guts of mammals and contributes to the break down of otherwise indigestible portions of the host’s diet, mainly plant material (Bernalier et al.). This break down of dietary polysaccharides and proteins is accomplished through fermentation in the anaerobic gut environment by microbial communities. These microbial communities are syntrophic, meaning that their interactions create a linked food web. Products of this metabolic food web are short chain fatty acids, such as acetate, other organic acids, and H2 and CO2 gases. The accumulation of H2 gas can actually inhibit the reoxidation of NADH, which reduces the amount of ATP and short chain fatty acids produced (Bain et al.). The metabolism of these short chain fatty acids is estimated to account for the production of 5 to 10% of the energy required by humans (McNeil, 1984). Organisms that consume H2 in the microbial gut community, such as Blautia hydrogenotrophica, play an important role in host metabolism, thus gaining a better understanding of these microbes could potentially lead to being able to manipulate the human energy balance (Bain et al.).
References
Bain, J., Faith, J., Gordon, J., Muehlbauer, M., Newgard, C., Rey, F., and Stevens, R. “Dissecting the in Vivo Metabolic Potential of Two Human Gut Acetogens.” Journal of Biological Chemistry, 2010. Volume 285. P. 22082-22090. Accessed April 18, 2011. <http://www.jbc.org/content/285/29/22082.full>
Bernalier, A., Collins, MD., Leclerc, M., Rochet, V., Willems, A. “Ruminococcus hydrogenotrophicus sp. nov., a new H2/CO2-utilizing acetogenic bacterium isolated from human feces.” Archives of Microbiology, 1996. Volume 166(3). p. 176-83. Accessed April 16, 2011. < http://www.springerlink.com/content/tt7hew38ngt4j9d7/>
Liu, C., Finegold, S., Song, Y., and Lawson, P. “Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydrogenotrophica comb. nov., Blautia luti comb. nov., Blautia producta comb. nov., Blautia schinkii comb. nov. and description of Blautia wexlerae sp. nov., isolated from human faeces.” International Journal of Systematic and Evollutionary Microbiology, 2008. Volume 58. p. 1896-1902. Accessed April 21, 2011. <http://ijs.sgmjournals.org/cgi/content/full/58/8/1896>
McNeil, N. “The contribution of the large intestine to energy supplies in man.” American Journal of Clinical Nutrition, 1984. Volume 39. p. 338-342. Accessed April 18, 2011. <http://www.ajcn.org/content/39/2/338.abstract?ijkey=3f1e8024ab51ee3f0e37493ec8bd86b448209b24&keytype2=tf_ipsecsha>
NCBI (National Center for Biotechnology Information). “Blautia hydrogenotrophica DSM 10507, whole genome shotgun sequence.” Accessed April 21, 2011.
<http://www.ncbi.nlm.nih.gov/nuccore/NZ_ACBZ00000000>
Williams YJ, Popovski S, Rea SM, Skillman LC, Toovey AF, Northwood KS, Wright AD. "A vaccine against rumen methanogens can alter the composition of archaeal populations." Applied and environmental Microbiology, 2009. Volume 75.7. p. 1860-1866. Accessed April 18, 2011. <http://www.ncbi.nlm.nih.gov/pubmed/19201957>
Author
Page authored by Megan Buhl and Blaze Budd, students of Prof. Jay Lennon at Michigan State University.
