Bovine spongiform encephalopathy: Difference between revisions
| Line 5: | Line 5: | ||
[[Image: | [[Image:BrainSection.jpg|thumb|400px|right|Figure 1 Image of a brain infected with BSE. http://www.cfsph.iastate.edu/DiseaseInfo/disease-images.php?name=bovine-spongiform-encephalopathy]] | ||
Transmissible spongiform encephalopathies are known as prion diseases, which can cause progressive neurological disorders. The most well known prion disease is bovine spongiform encephalopathy (mad cow disease). Mad Cow Disease was first found in cattle in England in 1986 causing hundreds of thousands of cattle to die. Between 1985-95, almost 1 million cattle were infected in the UK and about ¾ of those were fed to humans (Campbell 2006). Heavy restrictions were placed on cattle export throughout the world, with the heaviest restrictions occurring in Europe. The USDA also implemented stringent measures, which banned products from nations where BSE was found. While the disease has been very prevalent in recent decades, the exact method of transfer is still not understood by researchers. The disease is not believed to be a product of bacteria, viruses, parasites and fungus, but is believed to be the product of a misfolded prion protein in cattle (bseinfo). Prion proteins are commonly found throughout the body, but they can be transformed into the modified form due to an accumulation of the modified prion proteins, which can eventually lead to damage and disease in the brain. What makes this prion protein more complex is the complexity in killing it as it is able to survive heat, UV light, ionizing radiation and normal sterilization techniques (USDA 2015). The prion is also partially resistant to Proteinase K, a type of amino acid breakdown, whichs leads scientists to believe that the prion is not destroyed in the gastrointestinal tract (Campbell 2006). | Transmissible spongiform encephalopathies are known as prion diseases, which can cause progressive neurological disorders. The most well known prion disease is bovine spongiform encephalopathy (mad cow disease). Mad Cow Disease was first found in cattle in England in 1986 causing hundreds of thousands of cattle to die. Between 1985-95, almost 1 million cattle were infected in the UK and about ¾ of those were fed to humans (Campbell 2006). Heavy restrictions were placed on cattle export throughout the world, with the heaviest restrictions occurring in Europe. The USDA also implemented stringent measures, which banned products from nations where BSE was found. While the disease has been very prevalent in recent decades, the exact method of transfer is still not understood by researchers. The disease is not believed to be a product of bacteria, viruses, parasites and fungus, but is believed to be the product of a misfolded prion protein in cattle (bseinfo). Prion proteins are commonly found throughout the body, but they can be transformed into the modified form due to an accumulation of the modified prion proteins, which can eventually lead to damage and disease in the brain. What makes this prion protein more complex is the complexity in killing it as it is able to survive heat, UV light, ionizing radiation and normal sterilization techniques (USDA 2015). The prion is also partially resistant to Proteinase K, a type of amino acid breakdown, whichs leads scientists to believe that the prion is not destroyed in the gastrointestinal tract (Campbell 2006). | ||
Revision as of 19:33, 8 May 2015
By Kevin Pan
Overview
By Kevin Pan
Transmissible spongiform encephalopathies are known as prion diseases, which can cause progressive neurological disorders. The most well known prion disease is bovine spongiform encephalopathy (mad cow disease). Mad Cow Disease was first found in cattle in England in 1986 causing hundreds of thousands of cattle to die. Between 1985-95, almost 1 million cattle were infected in the UK and about ¾ of those were fed to humans (Campbell 2006). Heavy restrictions were placed on cattle export throughout the world, with the heaviest restrictions occurring in Europe. The USDA also implemented stringent measures, which banned products from nations where BSE was found. While the disease has been very prevalent in recent decades, the exact method of transfer is still not understood by researchers. The disease is not believed to be a product of bacteria, viruses, parasites and fungus, but is believed to be the product of a misfolded prion protein in cattle (bseinfo). Prion proteins are commonly found throughout the body, but they can be transformed into the modified form due to an accumulation of the modified prion proteins, which can eventually lead to damage and disease in the brain. What makes this prion protein more complex is the complexity in killing it as it is able to survive heat, UV light, ionizing radiation and normal sterilization techniques (USDA 2015). The prion is also partially resistant to Proteinase K, a type of amino acid breakdown, whichs leads scientists to believe that the prion is not destroyed in the gastrointestinal tract (Campbell 2006).
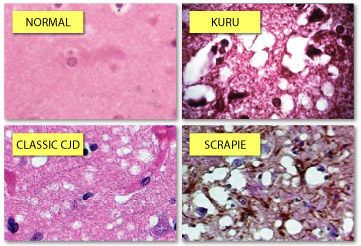
Other well known prion diseases are Kuru, which is a disease found among New Guinea natives, Gerstmann-Straussler-Scheinker disease and the variant Creutzfeldt-Jakob disease, which is believed to be caused by the ingestion of products tainted with BSE. Symptoms of CJD are dementia, memory loss, personality changes and hallucinations.
While the disease has appeared to be almost eradicated, it is still relevant to current research. Recent studies have shown a link between bovine prion disease and Alzheimer’s disease.
Prion Structure
Prions can be commonly found in all sorts of organisms including nonmammalisn organisms such as yeast and fungi. In Saccharomyces cerevisiae, the prions are composed, respectively, of Sup35p, a translation termination factor, and Ure2p, a transcriptional regulator involved in nitrogen metabolism (Nelson et al 2005). In a study done by Nelson et al (2005), they utilized X-ray crystallographic analysis to study amyloid polymers of a peptide derived from the N-terminal of Sup35p. The analysis showed that the structure consists of a pair of pair of β sheets running parallel to the fiber axis and are held together by hydrogen bonds. However, this prion differs in many ways from mammalian prions. In a mammal, a prion is a type of protein that triggers normal proteins in the brain to fold abnormally, which affect the nervous system in humans and animals. Harris et al (2006) studied the mammalian prion structure through the use of NMR spectroscopy and X-ray crystallographic analysis. They found that PrPC consists of a N-terminal region encoded by the residue region between 23-142 and a C-terminal region encoded by the residues 125-228. Further analysis showed that the C-terminal consists of of three α-helices (figure 2) and two short β-strands.
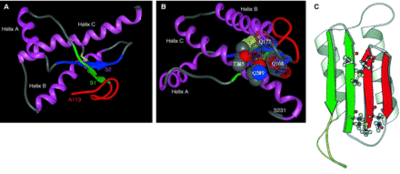
Hagiwara et al (2012) also showed that the N-terminus is supposed to be flexible in a normal prion protein. Prusiner (1997) believes it is the NH2 terminal where PrPSc binds to PrPC.
The tertiary structure of PrPSc has been a little more difficult to determine because it is difficult to determine the tendency of the proteins aggregating together. Models of PrPSc indicate that the main cause of the prion diseases is the refolding of the NH2 terminal helices into β-sheets.
Healthy mammals have normal prion proteins (PrPC) with have amino acid sequences that are highly conserved in mammalian species. This protein consists of 254 amino acids, which includes a signal peptide at the N-terminus, as well as the c-terminus. The protein also includes two asparagines for nitrogen glycosylation (Hagiwara 2013). Unlike other infectious agents, prions are unique in that they do not have nucleic acids (Korth et al 2001). Comparing the two proteins with each other can elicit several differences. Firstly, it was shown through infrared spectroscopy that the normal prion protein consists of roughly 40% α-helixes, whereas PrPSc was shown to have a higher β-sheet (54%) content than α-helices (21%) content. It is believed that this high β-sheet content is what makes the misfolded proteins pathogenic (Prusiner 1997). It was found that human prion diseases are closely related with two types of scrapie prion proteins that can be detected using a Western blot based on the size of PrPSc (Gambetti et al 2003).
Prion Diseases
All prion diseases in humans and nonhumans are very similar to one another and have been around for awhile. One of the earliest known prion diseases, “scrapie,” has been around since 1732. This degenerative disease affects the nervous systems of sheep and goats and is characterized by animals scraping off their fur or wool (figure 3) (bseinfo). Other symptoms of the disease are changes in behavior, tremors and locked muscles and a loss of movement. The sheep that are affected are generally between the ages of three and five years old and once they are infected, the animals generally die within 2 weeks to 6 months of onset. Dissections of afflicted sheep indicate the proteins’ presence throughout the nervous system and various important organs.

The more prevalent prion diseases are BSE and the diseases that affect humans.
Bovine Spongiform Encephalopathy
Mad cow disease was an epidemic in the 1980s and 1990s and was one of the worst prion diseases in agriculture history, which raised major concerns in human food and animal feed safety (Gray et al 2012). Cattle were found to suffer from body tremors, loss of limb mobility and aggressive behavior, which are symptoms similar to scrapie. BSE has an incubation period of anywhere 20 months up to the lifetime of the animal, but generally affects adult cattle between the ages of 3 to 6 (bseinfo).
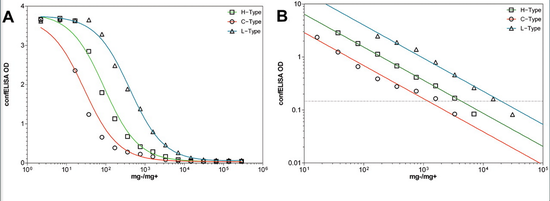
Due to the long incubation period of BSE with no apparent symptoms, diagnosis can be difficult. Cattle are put into the high-risk category if they display symptoms of ataxia, aggression and unexplained weight loss, therefore they must be put down in order to perform a biopsy on its brain. Common tests for this disease are ELISA (Figure 4), western blotting and lateral flow tests (Gray et al 2012). The cattle that display symptoms such as ataxia, aggression and unexplained weight loss are categorized into the high-risk category (Gray et al 2012). Currently, BSE can be divided into two strains: the typical BSE strain and the atypical BSE strain (CDC 2015). The typical BSE strain is the strain that is most common and led to the epidemic in the United Kingdom. The atypical strain is a distinct strain that may have risen spontaneously. Besides the United Kingdom, Japan has the highest number of cases of BSE outside of Europe.
The largest known epidemic of mad cow disease occurred in England. 177 people died after contracting variant Creutzfeldt-Jakob disease, whom are believed to have been caused by eating cattle infected with BSE. In 1992 and 1993, 36,680 and 34,370 cattle died due to this disease (Gray et al 2012). After 10 people had died of CJD in 1996, the British government finally acknowledged that people were getting sick off of a variant of BSE. Most cases of CDJ have occurred in the UK with nearly all of them happening between 1980-1996 (Campbell 2006). A 10-year ban on British beef was finally removed by the EU in 2006 to allow for exportation of British beef (euractiv 2006).
Human prion diseases
The most common type of prion diseases afflicting humans are Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker syndrome (GSS), and fatal familial insomnia (FFI). 10-15% of these disorders are caused by mutations to the PRNP gene on chromosome 20, which encode the prion protein (PrPC). The other 85-90% of cases are classified as sporadic or acquired (bseinfo). Prion diseases that are termed acquired prion diseases are due to exposure to PrPSc, which causes the disease.The Variant Creutzfeldt-Jakob disease is an example of an acquired disease because it can be obtained from eating cows that have been afflicted with BSE.
There are three variations of CJD disease: variant, sporadic and familial. Variant is caused by the consumption of infected food. Sporadic CJD is what causes the majority of CJD and occurs at random—the trigger for this form is currently unknown. Familial CJD is when a genetic mutation causes the formation of the abnormal prions. This disease has the largest impact in the 45 to 75-year old age group, with peak onset between 60 and 65 years (Collinge 2001). More importantly, this disease has a high mortality rate, with around 70% of those affected dying within 6 months. In about 1/3 of the cases, patients present symptoms of fatigue, depression, weight loss, headaches and insomnia. Analysis of people affected with variant Creutzfeldt-Jackob disease has shown that affected individuals were homozygous for methionine at PRNP codon 129. In a study conducted by Asante et al 2002, they were able to show that mice homozygous for methionine at 129 acquired the prion disease after inoculation with BSE. Amongst the Caucasian population, roughly 50% of the individuals are homozygous for M, about 35% are heterozygous for MV and the rest are homozygous for valine. There have been a little over 200 reported cases of vCJD since 1996 with the United Kingdom having the most cases of 177.
Another disease that affects humans that is similar to BSE is Kuru, which is a neurological disorder that is found in the Fore people of Papua New Guinea contracted. There was a peak mortality rate of 2%. After numerous studies, it is now understood that this disease was transferred among tribe members due to their funerary cannibalistic rituals. This disease was first noted in the mid 1950’s and was found long after cannibalistic rituals ended in that region. This disease is characterized by tremors, headaches, coordination problems and arm and leg pain. This disease primarily affected women and children because they were the primary participants in the funerary rituals, so many villages ended up becoming desolate of women (Mead et al 2009).
Control of Mad Cow Disease and its effect on the cattle industry
There has been a decline in cases of mad cow disease in recent years due to a ban on feeding cattle meat and bone meal. Besides feeding surveillance, important regulations are important for containing this disease. In England, a stringent control system was implemented that prevented any animals over 30 months old to be sold as human food and animal feed (Collinge et al 2001).
In the United States, prevention measures began in 1989 by banning import products from nations where BSE was found. In 1997, the FDA banned ruminant-based protein supplements that were being used as cattle feed. A USDA inspector will inspect all cattle that are sent out for consumption and any cattle suspected of having BSE are sent for further testing. Due to these stringent restrictions, BSE is prevalent in less than one infected cattle per 1 million (bseinfo). Additionally, most of the vCJD deaths have not occurred in the United States.
Because of the epidemic, different markets in recent decades around the world have gone through phases of banning and unbanning American beef. After a case of BSE was found in the US in 2003, Japan halted all imports of US beef. They resumed importation in 2005, but the ban was put back in place in 2006. Other countries followed suit—65 countries implemented similar restrictions on importing beef products from the US because they believed the US was not effectively monitoring their cow imports. As a result of all these restrictions, the amount of exported beef declined from 1.3 million metric tons in 2003 to a little over 300,000 metric tons in 2004. However, this value was able to increase to a little over 750,000 in 2007.
Other nations have experienced export regulations by other nations. Brazil was forced to stop exporting beef to Japan after a cow was claimed to have BSE in 2010.
Treatment
Treatment of prion diseases has thus far been very difficult due to the prions’ resilience towards common sterilization techniques. There have been compounds that inhibit the prion diseases when applied during the inoculation phase, but no treatments have been effective when administered before or during the onset of the disease (Korth et al 2001). Researchers have ideas about what might be effective. One idea is to interfere the conversion of PrPC into PrPSc because this would prevent the mass production of PrPSc. In order to do this, a drug could bind to PrPC to stabilize the structure, which would interfere with the transition to PrPSc. Another idea would be to destabilize the structure of PrPSc (Prusiner 1997). Prusiner also proposed the production of animals that do not replicate prions.
Other methods of treatment involve anti-prion antibodies that can cross the blood-brain barrier. In one study, the researchers were able to grow an anti-prion antibody that was capable of passing the blood-brain barrier in vitro; it also did not display any type of neurotoxic effects (Perry et al 1997).
In a study done by Baxter et al (2005), they were able to show the effectiveness of radio frequency gas plasma treatment against misfolded prion proteins. In this specific study, the surgical equipment that they had used were treated with gas-plasma to remove residual contamination and they found no apparent damage to the instruments. They were able to reduce residual contamination to levels undetectable by SEM.
Other studies have experimented with inhibits of cholesterol biosynthesis because it has been demonstrated before that PrPSc formation occurs in cholesterol-rich domains. However, it was determined that the level of cholesterol depletion required is not feasible for usage in animals (Korth et al 2001).
Recent Experiments
An issue that some medicines have is their ability to bass the blood-brain barrier, which restricts the ability for medicines to reach the central nervous system. There are studies that research which medicines can pass the barrier and inhibit the prions. One such method involves treating the abnormal prion (PrPSc) with tricyclicacridine and phenothiazine derivatives. Phenothiazine derivatives are compounds with a tricyclic scaffold and an aliphatic side chain that extends from the component (Korth et al 2001). Quinacrine, an example of a phenothiazine derivative, has been used as an antimaralial drug for over 60 years and is currently believed to be future of treatment for patients with variant CJD due to its high potency. To successfully inhibit PrPSc formation, the ideal amount of carbon atoms in the aliphatic side chain is around 3 to 4. This medicine also suggests the importance of the aliphatic side chain in inhibiting abnormal prion formation. In a specific experiment by Korth et al (2001), the researchers infected neuroblastoma cells with a strain of mouse-adapted scrapie prions. The researchers found that after the removal of quinacrine,the cells showed no infection after 3 weeks. This was proved by a western blot (figure 5).
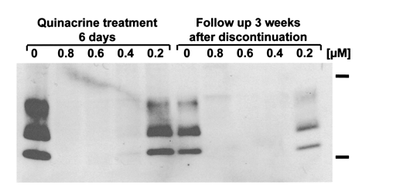
This experiment is significant because it shows that infected prion formation is inhibited. This bodes well for future research into therapies that can inhibit mutated prion growth and ideally clearance from cells. While this drug has a high potential for possible remedies, further research is needed because the actual mechanism for how these drugs act on PrPSc is still relatively unknown (Korth et al 2001). It must also be noted that this treatment takes a few days before it is effective. Other possible treatments include Congo red, which binds to the β-sheet structures and are believed to act nonspecifically on the PrPSc template by separating the infectious template (Korth et all 2001).
Recent News
Recently, a Texas man died and autopsies revealed that he died of mad cow disease. This was only fourth reported vCJD case in the world since 2012. Researchers are trying to determine how he could have been infected with this disease. They found out that the man was a US citizen who was born outside of the United States, which allowed him to be exposed to the BSE/vCJD agent in either Kuwait, Lebanon or Russia (CDC 2014). The Canadian Food inspection Agency also recently confirmed another case of mad cow disease in a cow from Alberta (CDC 2014).
Other recent research has shown a correlation between bovine spongiform encephalopathy and Alzheimer’s disease. There appears to be a link to the prion proteins in BSE and Alzheimer’s disease (NPR 2009). This can lead to studies that could possibly lead to a treatment for Alzheimer’s or other neurodegenerative disorders such as ALS and Parkinson’s.
Works Cited
1). Baxter, H., Campbell, G., Whittaker, A., Jones, C., Aitken, A., Simpson, A., Casey, M., Bountiff, L., Gibbard, L., and Baxter, R., 2005, Elimination of transmissible spongiform encephalopathy infectivity and decontamination of surgical instruments by using radio-frequency gas-plasma treatment, Journal General Virology, vol 86, pg 2393-2399,[1]
2). BSE info. [2]
3). Campbell, Peter N. "Mad cow disease." Acta Biologica Szegediensis 50.3-4 (2006): 89-95.[3]
4). Centers for Disease Control and Prevention, 2014, Confirmed Variant Creutzfeldt-Jakob Disease (variant CJD) Case in Texas. [4]
5). Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519-50. doi: 10.1146/annurev.neuro.24.1.519. PubMed PMID: 11283320.[5]
6). Gambetti P, Kong Q, Zou W, Parchi P, Chen SG. Sporadic and familial CJD: classification and characterisation. Br Med Bull. 2003;66:213-39. PubMed PMID: 14522861.[6]
7). Gray JG, Dudas S, Graham C, Czub S. Performance analysis of rapid diagnostic tests on atypical bovine spongiform encephalopathy. J Vet Diagn Invest. 2012;24(5):976-80. doi: 10.1177/1040638712455325. PubMed PMID: 22855378.[7]
8). Hagiwara, K., Hara, H., and Hanada, K., 2013, Species-barrier phenomenon in prion transmissibility from a viewpoint of protein science, Journal Biochemistry, vol 153, pg 139-145,[8]
9). Harris DA, True HL. New insights into prion structure and toxicity. Neuron. 2006;50(3):353-7. doi: 10.1016/j.neuron.2006.04.020. PubMed PMID: 16675391.[9]
10). Korth C, May BC, Cohen FE, Prusiner SB. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc Natl Acad Sci U S A. 2001;98(17):9836-41. doi: 10.1073/pnas.161274798. PubMed PMID: 11504948; PubMed Central PMCID: PMCPMC55539.[10]
11). Mead S, Whitfield J, Poulter M, Shah P, Uphill J, Campbell T, et al. A novel protective prion protein variant that colocalizes with kuru exposure. N Engl J Med. 2009;361(21):2056-65. doi: 10.1056/NEJMoa0809716. PubMed PMID: 19923577.[11]
12). Perry, V. H., et al. "The blood-brain barrier and the inflammatory response." Molecular medicine today 3.8 (1997): 335-341.[12]
13). [13]
14). USDA. “Bovine Spongiform Encephalopathy.” [14]
