Burkholderia pseudomallei infection


Etiology/Bacteriology
Taxonomy
| Domain = Bacteria
| Phylum = Proteobacteria
| Class = Betaproteobacteria
| Order = Burkholderiales
| Family = Burkholdiaceae
| Genus = Burkholderia
| species = B. pseudomallei
Description
Burkholderia pseudomallei is a gram-negative, rod-shaped, motile, soil-dwelling bacterium [2] . B. pseudomallei is the causative agent for Melioidosis, which is also referred to as Whitemore’s disease. The organism is found in soil and water in tropical climates such as, Southeast Asia and Northern Australia. Infection is often hard to diagnose because of the wide range of symptoms that can present all over the body. Symptoms are similar to a range of diseases and are often misdiagnosed, which can lead to fatality. Interest in the organism has recently spiked due to its potential as a bioterrorism weapon. Melioidosis is classified by the CDC as a category B bioterrorism concern. Classification in category B is based on: ease of dispersal, moderate morbidity rates and low mortality rates, and the requirement of and increased diagnostic capacity and disease surveillance by the Center for Disease Control. [3] Other diseases classified as a category B bioterrorism threat include: typhus fever and cholera.
Pathogenesis
Transmission
Transmission of B. pseudomallei regularly occurs through inhalation, ingestion or direct contact with contaminated soil or water. Innoculation of the organism usually occurs through skin abrasions. [4] Human-to-human transmission is very rare. Humans are not the only susceptible host to B. pseudomallei, other animals include: sheep, goats, swine, horses, cats, dogs and cattle. [5] There have been few cases of zoonotic transmission, it is often transmitted through exposure to skin lesions by direct contact with infected animal either through meat or milk. [6] Sexual transmission and vertical transmission have also been suggested in some cases but this is not common.
Infectious dose and incubation period
The infectious dose of B. pseudomallei is 10 colony forming units. [7] The incubation period of the organism varies, due to the type of infection and severity of infection. It ranges from one day to many years. [5] Generally, symptoms will appear two to four weeks after exposure to the pathogen. [5]
Epidemiology
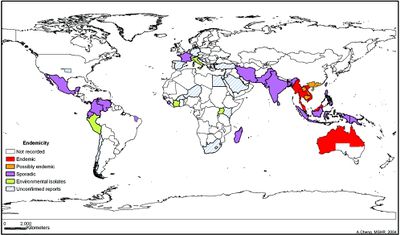
Although it is possible for a healthy person to acquire Melioidosis, more than 70% of cases occur in persons with other illnesses, such as renal disease and diabetes. [6] Northern Australia and Southeast Asia are considered endemic areas for B. pseudomallei infections.[8] However, cases have also been reported in areas such as South Pacific, Africa, India, and the Middle East. [4] Around 75% of reported cases of Melioidosis occur during the rainy seasons.[9] In the United States, confirmed cases of B. pseudomallei infection range from 0-5 a year and are usually a result of travelers or immingrants. [5] Mortality of Melioidosis varies based on severity, type of infection, and access to health care. In the case of acute severe melioidosis the fatality rate is 30 to 47%. [6] In cases of untreated septicemia, the mortality rate is greater than 90%; however when treated, fatality drops to 40-75%. [6] When septic shock develops in the patient, the fatality rate is approximately 95%. [6] The mortality rates of Melioidosis are often affected by the difficulty to diagnose the disease due to its large range of symptoms. However, in areas with more adequate health care, like portions of Australia, mortality rate for patients with B. pseudomallei is approximately 20%. [6] Melioidosis can be developed at any age however, the average age of development is between 40 and 60 years. [9]
Virulence Factors
B. pseudomallei is a persistent infection due its ability to survive in many harsh conditions such as, nutrient deficiency, variable pH ranges, disinfectants and antiseptic treatments, many antibiotics, and extreme temperatures. [8] The organism is well adapted to many different types of hosts and is able to resist many types of cell defenses such as cationic peptidases and lysosomal defensins. [8] The organism is able to survive within many of the phagocytic cells in the host’s immune system, like neutrophils and macrophages. Many virulence factors in B. pseudomallei have been identified such as, capsules, Type IV pilin, Type II and III secretion systems and more. With the variability of the disease’s symptoms it has been suggested that virulence factors may vary with severity and type of infection. [10]
Capsule
The capsule, a group 3 capsular polysaccharide, is highly advantageous in B. pseudomallei because it reduces the ability of complement factor Cb3 to opsonize the surface of the bacterium therefore reducing the likelihood of phagocytosis. [11] The capsular polysaccharide appears to aid in environmental protection, host immune evasion and attachment to the epithelial cells. [8]
Secretion Systems
The Type II secretion systems are required for the secretion of products such as protease, lipase, phospholipase C, hemolysin, and lecithinase.[8] However, these products have shown to play a very minor role in the pathogenicity of the organism. [11] The Type III secretion systems are believed to produce products such as BopE and Bsa which are important in host cell invasion. This was shown using mutated organisms without the Bsa and BopE system. The B. pseudomallei organism was unable to access the cell’s actin which, suggests that these products are important for endosomal membrane lysis.[8] It has also been suggested that the type III secretion systems allow for the evasion of endocytic vesicles. [11]
PilA
The Type IV Pilin, PilA, is thought to be important in the adherence of B. pseudomallei. Type IV pili are important in the virulence of my gram-negative bacterial species. [12] B. pseudomallei adheres to human epithelial cells however, the mechanism by which is does so is still largely unknown. [12] It has been suggested that the PilA gene would be an important target in the future attempts of the development of a vaccine against melioidosis. [12]
Clinical Features

According the Center for Disease Control, there are four types of B. pseudomallei infections: localized, pulmonary, bloodstream, or disseminated. Localized infection usually presents as ulcer, nodule or skin abscess. These types of infections usually occur from the bacterium breaching through a break in the skin. Localized infection can result in fever or muscle aches in the area. Pulmonary infections are the most common form of infection and are often the hardest to diagnose because they can present as mild bronchitis or severe pneumonia. They are characterized by high fever, headache, anorexia and muscle soreness. Chest pain is also common with a pulmonary infection however; a nonproductive or productive cough with normal sputum is a key characteristic for this type of infection. [5] In a bloodstream infection, patients may experience abdominal discomfort, joint pain, muscle tenderness, and disorientation. Abscesses may be found throughout the body especially in the liver, spleen or prostate. [5] With this type of infection symptoms are rapidly onset and patients with renal disease and diabetes are more susceptible to this type of infection.[5] In these patients, a bloodstream infection by B. pseudomallei usually results in septic shock. In a disseminated infection abscesses are common in the liver, spleen, lung and prostate. Other areas of the body included in this type of infection are the joints, bones, viscera, lymph nodes, skin and brain. Symptoms of this infection include: fever, weight loss, stomach or chest pain, muscle or joint pain, headaches, and seizures. [5] Infections by B. pseudomallei are often very serious as they can present in many different ways and can develop into kidney disease, blood disease, heart disease and other fatal disorders. [2]
Diagnosis
Diagnosis of Melioidosis is best achieved through the isolation of the organism from a sample taken from the blood, sputum, skin lesion, abscess, or urine. [5] The bacteria can be isolated on the Ashdown medium first described by L.R. Ashdown in 1979. [8] The medium contains: tryptase soy agar with glycerol, crystal violet, natural red, gentamicin and colistin. Gentamicin prevents the growth of other organisms, which allows the sample to be taken from non-sterile sites on the patient. [8] Detection of an antibody response to the bacteria is also a form of diagnosis; however isolation of Burkholderia pseudomallei is more commonly used.
Treatment
The course of treatment for Melioidosis is subject to the severity and type of infection. The most common form of treatment begins with intravenous antimicrobial therapy for 10-14 days, followed directly by 3-6 months of oral antimicrobial therapy. [5] Two of the most common intravenous antimicrobial medications used are ceftazidime administered every 6-8 hours or meropenem administered every 8 hours. Trimethoprim-sulfamethoxazole or doxycycline may both be used for the oral antimicrobial therapy. [5] Alternative treatments are considered in cases of penicillin allergies. Treatment of B. pseudomallei is often dependent on the severity of the infection, as well as, the immunological health of the patient. Patients with diabetes or renal disease are often more susceptible to infection and therefore require a different course of treatment. In terms of respiratory infection, if abscesses develop on the lung after 6 months of positive culture on the lung, a lobectomy is performed to remove the abscess. [4]
Prevention
Infection by Burkholderia pseudomallei in the endemic regions can be prevented by avoiding contact with soil and standing water, often the modes of transmission. Persons with open skin wounds, diabetes, or chronic renal disease are at an increased risk for infection. In endemic areas, agricultural workers should wear boots to prevent infection through the feet and calf. [5] Health care professionals who may encounter the disease should take normal precautions.
Vaccination
Currently there is no vaccine for infection by B. pseudomallei. However, there has been recent research in the advantages of a development of an immunization to Melioidosis in response to bioterrorism threats. [13] The development of a vaccine has also been encouraged as a protective agent in endemic areas, such as Northern Australia and Asia. In these areas, the vaccine would be used predominantly in the high-risk population, such as those suffering from renal disease or diabetes. [13] Although no vaccine has been approved for humans, the animal models have shown promising results.
Host Immune Response
There is an abundance of research into the mechanisms by which B. pseudomallei is able to evade the host immune system. Research has shown the infection is resistant to human serum by reducing the ability of complement protein C3b to opsonize the pathogen. [14] The organism is also able to evade the membrane attack complex. Research has also shown B. pseudomallei can survive within a phagocyte by evading the fusion of the phagosome-lysosome complex and destroy the membrane of the phagosome as quickly as 15 minutes after ingestion of the bacteria. [8] Macrophages infected with B. pseudomallei were shown to have poor expression of nitric oxide and TNF-alpha. [14] This suggests that Melioidosis infection significantly diminishes the innate immune response. Furthermore, cases in which the infection has a longer incubation period, which have been reported for as long as 62 years, would suggest the bacteria’s ability to remain latent in the body. [6] Establishment of latency within the body has suggested failure of the adaptive immune response in clearing the infection however, the mode by which it evades the adaptive immune response is still unknown.
References
1 University of Oklahoma Education Abroad.
2 "Burkholderia pseudomallei" Keara Pringle
3 "Bioterrorism Agents/Diseases By Category" Center for Disease Control
4 "Melioidosis (Whitmore's Disease)" MedicineNet.com
5 "Meliodosis" Center for Disease Control
6 "Melioidosis, pseudoglanders, Whitemore's Disease" Iowa State University, The Center for Food Security and Public Health
7 "Burkholderia pseudomallei" Wasworth.org
8 "Melioidosis: Epidemiology, Pathophysiology and Management" Cheng, Allen C. and Currie, Bart J. Clinical Microbiology Reviews April 2005: 18(2): 383-416
9 "Melioidosis: a clinical overview" Limmathurotsakul, D, Peacock, SJ. May 9 2011
10 "Variable Virulence Factors in Burkholderia pseudomallei(Malioidosis) Associated with Human Disease" Sarovich, D.S., Price, E.P., Webb, J.R., Ward, L.M., Voutsinos, M.Y., Tuanyok, A., Mayo, M., Kaestli, M., Currie, B. J., PLoS ONE 9(3): e91682.
11 "Chapter 7: Melioidosis" Vietri, Nicholas J. MD and Deshazer, David PHD. Medical Aspects of Biological Warfare
12 "A Type IV Pilin, PilA, Contributes to Adherence of Burkholderia pseudomallei and Virulence in Vivo" Essex-Lopresti, A. E, Boddey, J. A, Thomas, R., Smith, M.P., Hartley, M.G., Atkins, T., Brown, N.T., Tsang, C.H., Peak, I. R. A., Hill, J., Beacham, I.R., Titball., Infection and Immunity. Feb 2005; 73 (2): 1260-1264.
13 "Melioidosis Vaccines: A Systematic Review and Appraisal of the Potential to Exploit Biodefense Vaccines for Public Health Purposes". Peacock, S.J., Limmathurotsakul, D., Lubell, Y., Koh, G. C. K. W., White, L.J., Day, N. P. J., Titball, R. W., PLOS Neglected Tropical Diseases. Jan 2012. 6(1): e1488.
14 "Interaction between Burkholderia pseudomallei and the Host Immune Response: Sleeping with the Enemy?" Gan, Y. The Journal of Infectious Diseases. 192 (10): 1845-1850.
Created by Rachel Garrison, student of Tyrrell Conway at the University of Oklahoma.
