CTXφ Bacteriophage: Difference between revisions
m (→Conclusion) |
mNo edit summary |
||
| Line 2: | Line 2: | ||
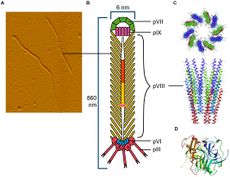
[[Image:Filamentous_phage.jpg|thumb|230px|right|<b>Figure 1:</b> Filamentous phage(s) under an electron microscope (left), an artist's rendition, and some computer-generated models of varying proteins found in the head, neck, and tail fibers.<ref name = Gagic>Gagic, D., Ciric M., Wen W., Ng F., Rakonjac J. (2016). "Exploring the Secretomes of Microbes and Microbial Communities Using Filamentous Phage Display." Frontiers in Microbiology, 7:429. https://doi.org/10.3389/fmicb.2016.00429.</ref> From <i>Gagic D. et al. (2016)</i>.]] | [[Image:Filamentous_phage.jpg|thumb|230px|right|<b>Figure 1:</b> Filamentous phage(s) under an electron microscope (left), an artist's rendition, and some computer-generated models of varying proteins found in the head, neck, and tail fibers.<ref name = Gagic>Gagic, D., Ciric M., Wen W., Ng F., Rakonjac J. (2016). "Exploring the Secretomes of Microbes and Microbial Communities Using Filamentous Phage Display." Frontiers in Microbiology, 7:429. https://doi.org/10.3389/fmicb.2016.00429.</ref> From <i>Gagic D. et al. (2016)</i>.]] | ||
The CTXφ bacteriophage (or sometimes written as CTX<i>phi</i> bacteriophage) is a lysogenic, filamentous, single-stranded DNA (ssDNA) phage that is responsible for turning the previously non-infectious <i>Vibrio cholerae</i> into a highly pathogenic microbe that causes cholera in humans.<ref name = Davis>Davis, B. M., Kimsey, H. H., Chang, W., & Waldor, M. K. (1999). "The Vibrio cholerae O139 Calcutta bacteriophage CTXφ is infectious and encodes a novel repressor." <i>Journal of Bacteriology</i>, 181(21), 6779-6787. https://www.frontiersin.org/articles/10.3389/fmicb.2016.00429/full</ref><ref name = Ochman>Ochman, H., Lawrence, J. & Groisman, E. (2000). "Lateral gene transfer and the nature of bacterial innovation." <i>Nature</i>, 405, 299–304. https://doi.org/10.1038/35012500.</ref><ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> This particular bacteriophage,<i>Vibrio virus</i> CTX<i>phi</i> belongs to the family <i>Inoviridae</i> and the genus <i>Affertcholeramvirus</i>. These phages are composed of only 5 viral proteins and are between 1 and 2 microns in length.<ref name = Mai-Prochnow>Mai-Prochnow, A., Hui, J., Kjelleberg, S., Rakonjac, J., McDougald, D., Rice, S. (2015). "Big things in small packages: the genetics of filamentous phage and effects on fitness of their host." <i>FEMS Microbiology Reviews, Volume 39, Issue 4</i>, Pages 465–487, https://doi.org/10.1093/femsre/fuu007</ref> They resemble something like a cooked spaghetti noodle. (See Figure 1 at right). | The CTXφ bacteriophage (or sometimes written as CTX<i>phi</i> bacteriophage) is a lysogenic, filamentous, single-stranded DNA (ssDNA) phage that is responsible for turning the previously non-infectious <i>Vibrio cholerae</i> into a highly pathogenic microbe that causes cholera in humans.<ref name = Davis>Davis, B. M., Kimsey, H. H., Chang, W., & Waldor, M. K. (1999). "The Vibrio cholerae O139 Calcutta bacteriophage CTXφ is infectious and encodes a novel repressor." <i>Journal of Bacteriology</i>, 181(21), 6779-6787. https://www.frontiersin.org/articles/10.3389/fmicb.2016.00429/full</ref><ref name = Ochman>Ochman, H., Lawrence, J. & Groisman, E. (2000). "Lateral gene transfer and the nature of bacterial innovation." <i>Nature</i>, 405, 299–304. https://doi.org/10.1038/35012500.</ref><ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> This particular bacteriophage,<i>Vibrio virus</i> CTX<i>phi</i> belongs to the family <i>Inoviridae</i> and the genus <i>Affertcholeramvirus</i>. These phages are composed of only 5 viral proteins and are between 1 and 2 microns in length.<ref name = Mai-Prochnow>Mai-Prochnow, A., Hui, J., Kjelleberg, S., Rakonjac, J., McDougald, D., Rice, S. (2015). "Big things in small packages: the genetics of filamentous phage and effects on fitness of their host." <i>FEMS Microbiology Reviews, Volume 39, Issue 4</i>, Pages 465–487, https://doi.org/10.1093/femsre/fuu007</ref> They resemble something like a cooked spaghetti noodle. (See Figure 1 at right). | ||
<br> | <br><br> | ||
==Genetic Material== | ==Genetic Material== | ||
[[Image:CTX_genome.jpg|thumb|200px|left|<b>Figure 2:</b> A schematic representation of the CTXφ genome.<ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> From <i>Boyd (2010)</i>.]] | [[Image:CTX_genome.jpg|thumb|200px|left|<b>Figure 2:</b> A schematic representation of the CTXφ genome.<ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> From <i>Boyd (2010)</i>.]] | ||
The CTXφ phage can be identified in its host in both its replicative form (when the bacteriophage is in lysogeny, or the lytic cycle), and in its non-replicative form, which is most common. It is in fact extremely rare to find or identify a CTXφ bacteriophage outside a host. This ssDNA phage, when in its lysogenic cycle, integrates its own genetic material in such a way as to form the most stable lysogens.<ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> The average size of a CTXφ genome is about 6.9 to 7 kb long and consists of two sections; the RS2 region and the core region.<ref name = Fan>Fan, F., Kan, B.(2015). "Survival and proliferation of the lysogenic bacteriophage CTXφ in <i>Vibrio cholerae</i>." Virologica Sinica. 30, 19–25. https://doi.org/10.1007/s12250-014-3550-7</ref> The core contains the genes that code for the Cholera toxin as well as proteins that are assumed to form the outside coat of the phage. The RS2 region is made up of genes that govern DNA synthesis and regulation as well as integration into host cells.<ref name = Fan>Fan, F., Kan, B.(2015). "Survival and proliferation of the lysogenic bacteriophage CTXφ in <i>Vibrio cholerae</i>." Virologica Sinica. 30, 19–25. https://doi.org/10.1007/s12250-014-3550-7</ref> <br> | The CTXφ phage can be identified in its host in both its replicative form (when the bacteriophage is in lysogeny, or the lytic cycle), and in its non-replicative form, which is most common. It is in fact extremely rare to find or identify a CTXφ bacteriophage outside a host. This ssDNA phage, when in its lysogenic cycle, integrates its own genetic material in such a way as to form the most stable lysogens.<ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> The average size of a CTXφ genome is about 6.9 to 7 kb long and consists of two sections; the RS2 region and the core region.<ref name = Fan>Fan, F., Kan, B.(2015). "Survival and proliferation of the lysogenic bacteriophage CTXφ in <i>Vibrio cholerae</i>." Virologica Sinica. 30, 19–25. https://doi.org/10.1007/s12250-014-3550-7</ref> The core contains the genes that code for the Cholera toxin as well as proteins that are assumed to form the outside coat of the phage. The RS2 region is made up of genes that govern DNA synthesis and regulation as well as integration into host cells.<ref name = Fan>Fan, F., Kan, B.(2015). "Survival and proliferation of the lysogenic bacteriophage CTXφ in <i>Vibrio cholerae</i>." Virologica Sinica. 30, 19–25. https://doi.org/10.1007/s12250-014-3550-7</ref> <br><br> | ||
==Infection, Replication & Release from Host Cell== | ==Infection, Replication & Release from Host Cell== | ||
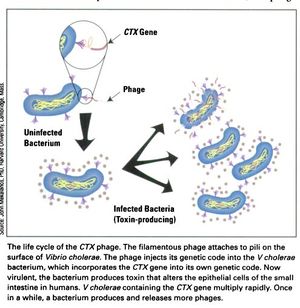
[[Image:CTX_Phage_life_cycle.jpg|thumb|right|<b>Figure 3:</b> The life cycle of the CTXφ Bacteriophage with <i>Vibrio cholerae</i> as its host.]] | [[Image:CTX_Phage_life_cycle.jpg|thumb|right|<b>Figure 3:</b> The life cycle of the CTXφ Bacteriophage with <i>Vibrio cholerae</i> as its host.]] | ||
In a process known as lysogenic phage conversion, the CTXφ bacteriophage integrates, among others, its <i>ctxAB</i> genes into its host, <i>Vibrio cholerae</i>. The <i>ctxAB</i> genes code for a type of exotoxin, called Cholera toxin or just "CT," that causes <i> V. cholerae</i> to switch from being nonpathogenic to highly virulent. For those infected, it is also the primary cause of the large amounts of watered-down diarrhea—cholera's main symptom.<ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> After finding a live host and undergoing CTXφ particle adsorption (see Figure 3 at right) to the <i>V. cholerae</i> cell wall, viral single-stranded DNA is then injected into the cell cytoplasm and a complementary strand of DNA is synthesized in order to form a circular plasmid, pCTX,<ref name = Waldor>Waldor, M., Davis, B. M. (2003). "Filamentous phages linked to virulence of Vibrio cholerae," <i>Current Opinion in Microbiology,Volume 6, Issue 1</i>, Pages 35-42, ISSN 1369-5274. https://doi.org/10.1016/S1369-5274(02)00005-X. (http://www.sciencedirect.com/science/article/pii/S136952740200005X)</ref>which then integrates into the <i>V. cholerae</i> genome at a site-specific attachment location on one or both chromosomes of <i> V. cholerae</i>.<ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> Because the CTXφ phage, like all bacteriophages, only injects its genome, or, more commonly, its <i>prophage</i>, the CTXφ integration makes use of many of the host cell's enzymes. An integrase enzyme is not present in the CTXφ genome, so it recruits XerC and XerD, two tyrosine recombinases present in <i>V. cholerae</i>, to catalyze this merge.<ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> Normally, XerC and XerD fix chromosome dimers in eubacteria.<ref name = Sherratt>Sherratt, D. J., Søballe, B., Barre, F. X., Filipe, S., Lau, I., Massey, T., & Yates, J. (2004). "Recombination and chromosome segregation." Philosophical transactions of the Royal Society of London. Series B, <i>Biological sciences</i>, 359(1441), 61–69. https://doi.org/10.1098/rstb.2003.1365</ref> CTXφ prophages usually integrate as tandem prophages,<ref name = McLeod>McLeod, S. M., Kimsey, H. H., Davis, B. M., Waldor, M. K. (2005). "CTXφ and Vibrio cholerae: exploring a newly recognized type of phage–host cell relationship." Microreview, 57 (2), 347–356. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2958.2005.04676.x.</ref> getting replicated whenever the host cell's genome does. After sufficient time has passed and enough proteins and genetic material has been packaged, he phage proteins are localized to the inner membrane prior to forming virions.<ref name = McLeod>McLeod, S. M., Kimsey, H. H., Davis, B. M., Waldor, M. K. (2005). "CTXφ and Vibrio cholerae: exploring a newly recognized type of phage–host cell relationship." Microreview, 57 (2), 347–356. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2958.2005.04676.x.</ref> Like many other filamentous phages, once emergence from the host cell occurs, it does not require lysis of the host's membrane. It is more of a secretion.<ref name = Faruque>Faruque, S. M., & Mekalanos, J. J. (2012). "Phage-bacterial interactions in the evolution of toxigenic <i>Vibrio cholerae</i>." <i>Virulence</i>, 3(7), 556–565. https://doi.org/10.4161/viru.22351</ref> Secretion of CTXφ phages from the bacterial cell occurs through a host‐encoded channel, EpsD.<ref name = McLeod>McLeod, S. M., Kimsey, H. H., Davis, B. M., Waldor, M. K. (2005). "CTXφ and Vibrio cholerae: exploring a newly recognized type of phage–host cell relationship." Microreview, 57 (2), 347–356. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2958.2005.04676.x.</ref> | In a process known as lysogenic phage conversion, the CTXφ bacteriophage integrates, among others, its <i>ctxAB</i> genes into its host, <i>Vibrio cholerae</i>. The <i>ctxAB</i> genes code for a type of exotoxin, called Cholera toxin or just "CT," that causes <i> V. cholerae</i> to switch from being nonpathogenic to highly virulent. For those infected, it is also the primary cause of the large amounts of watered-down diarrhea—cholera's main symptom.<ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> After finding a live host and undergoing CTXφ particle adsorption (see Figure 3 at right) to the <i>V. cholerae</i> cell wall, viral single-stranded DNA is then injected into the cell cytoplasm and a complementary strand of DNA is synthesized in order to form a circular plasmid, pCTX,<ref name = Waldor>Waldor, M., Davis, B. M. (2003). "Filamentous phages linked to virulence of Vibrio cholerae," <i>Current Opinion in Microbiology,Volume 6, Issue 1</i>, Pages 35-42, ISSN 1369-5274. https://doi.org/10.1016/S1369-5274(02)00005-X. (http://www.sciencedirect.com/science/article/pii/S136952740200005X)</ref>which then integrates into the <i>V. cholerae</i> genome at a site-specific attachment location on one or both chromosomes of <i> V. cholerae</i>.<ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> Because the CTXφ phage, like all bacteriophages, only injects its genome, or, more commonly, its <i>prophage</i>, the CTXφ integration makes use of many of the host cell's enzymes. An integrase enzyme is not present in the CTXφ genome, so it recruits XerC and XerD, two tyrosine recombinases present in <i>V. cholerae</i>, to catalyze this merge.<ref name = Boyd>Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107</ref> Normally, XerC and XerD fix chromosome dimers in eubacteria.<ref name = Sherratt>Sherratt, D. J., Søballe, B., Barre, F. X., Filipe, S., Lau, I., Massey, T., & Yates, J. (2004). "Recombination and chromosome segregation." Philosophical transactions of the Royal Society of London. Series B, <i>Biological sciences</i>, 359(1441), 61–69. https://doi.org/10.1098/rstb.2003.1365</ref> CTXφ prophages usually integrate as tandem prophages,<ref name = McLeod>McLeod, S. M., Kimsey, H. H., Davis, B. M., Waldor, M. K. (2005). "CTXφ and Vibrio cholerae: exploring a newly recognized type of phage–host cell relationship." Microreview, 57 (2), 347–356. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2958.2005.04676.x.</ref> getting replicated whenever the host cell's genome does. After sufficient time has passed and enough proteins and genetic material has been packaged, he phage proteins are localized to the inner membrane prior to forming virions.<ref name = McLeod>McLeod, S. M., Kimsey, H. H., Davis, B. M., Waldor, M. K. (2005). "CTXφ and Vibrio cholerae: exploring a newly recognized type of phage–host cell relationship." Microreview, 57 (2), 347–356. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2958.2005.04676.x.</ref> Like many other filamentous phages, once emergence from the host cell occurs, it does not require lysis of the host's membrane. It is more of a secretion.<ref name = Faruque>Faruque, S. M., & Mekalanos, J. J. (2012). "Phage-bacterial interactions in the evolution of toxigenic <i>Vibrio cholerae</i>." <i>Virulence</i>, 3(7), 556–565. https://doi.org/10.4161/viru.22351</ref> Secretion of CTXφ phages from the bacterial cell occurs through a host‐encoded channel, EpsD.<ref name = McLeod>McLeod, S. M., Kimsey, H. H., Davis, B. M., Waldor, M. K. (2005). "CTXφ and Vibrio cholerae: exploring a newly recognized type of phage–host cell relationship." Microreview, 57 (2), 347–356. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2958.2005.04676.x.</ref> | ||
<br> | <br><br> | ||
==CT & non-CT Toxins== | ==CT & non-CT Toxins== | ||
| Line 18: | Line 18: | ||
===CT Toxins=== | ===CT Toxins=== | ||
The epithelial cells of the small intestine contain a receptor called ganglioside GM1. This is a the natural biological receptor for cholera toxin.<ref name = Holmgren>Holmgren, J. (1981). "Actions of cholera toxin and the prevention and treatment of cholera." <i>Nature</i>, 292(5822), 413-417.</ref> Once infected with the CTXφ bacteriophage, <i>Vibrio cholerae</i> has the ability to potentially create new ganglioside GM1 receptors in the environment that the bacteria inhabits through an enzyme called sialidase. However, this doesn't seem to work well in humans, as a study in 1976 saw that no new GM1 receptors were created in the cells of intestinal epithelium.<ref name = Holmgren>Holmgren, J. (1981). "Actions of cholera toxin and the prevention and treatment of cholera." <i>Nature</i>, 292(5822), 413-417.</ref> Human intestines seem to have some sort of defenses put in place against enzymatic attacks like those from <i>V. cholerae</i>'s sialidase. | The epithelial cells of the small intestine contain a receptor called ganglioside GM1. This is a the natural biological receptor for cholera toxin.<ref name = Holmgren>Holmgren, J. (1981). "Actions of cholera toxin and the prevention and treatment of cholera." <i>Nature</i>, 292(5822), 413-417.</ref> Once infected with the CTXφ bacteriophage, <i>Vibrio cholerae</i> has the ability to potentially create new ganglioside GM1 receptors in the environment that the bacteria inhabits through an enzyme called sialidase. However, this doesn't seem to work well in humans, as a study in 1976 saw that no new GM1 receptors were created in the cells of intestinal epithelium.<ref name = Holmgren>Holmgren, J. (1981). "Actions of cholera toxin and the prevention and treatment of cholera." <i>Nature</i>, 292(5822), 413-417.</ref> Human intestines seem to have some sort of defenses put in place against enzymatic attacks like those from <i>V. cholerae</i>'s sialidase. | ||
<br> | |||
<br> | <br> | ||
====Non-CT Toxins==== | ====Non-CT Toxins==== | ||
Recent research suggests that there are perhaps two toxins (other than CT) that are produced from genes of the CTXφ genome. The first of these is accessory cholera enterotoxin, or Ace. Ace is currently thought to be a minor coat protein of CTXφ, though the process by which the toxin could be released from the protein coat is not yet known to scientists. The second non-CT toxin encoded within the CTXφ bacteriophage genome is zonula occludens toxin, or Zot. Zot, though absolutely essential for the production of the CTXφ virion, is not actually present in the phage particle. The part that Ace and Zot play in the virulence of cholera is still unknown.<ref name = Waldor>Waldor, M., Davis, B. M. (2003). "Filamentous phages linked to virulence of Vibrio cholerae," <i>Current Opinion in Microbiology,Volume 6, Issue 1</i>, Pages 35-42, ISSN 1369-5274. https://doi.org/10.1016/S1369-5274(02)00005-X. (http://www.sciencedirect.com/science/article/pii/S136952740200005X)</ref> | Recent research suggests that there are perhaps two toxins (other than CT) that are produced from genes of the CTXφ genome. The first of these is accessory cholera enterotoxin, or Ace. Ace is currently thought to be a minor coat protein of CTXφ, though the process by which the toxin could be released from the protein coat is not yet known to scientists. The second non-CT toxin encoded within the CTXφ bacteriophage genome is zonula occludens toxin, or Zot. Zot, though absolutely essential for the production of the CTXφ virion, is not actually present in the phage particle. The part that Ace and Zot play in the virulence of cholera is still unknown.<ref name = Waldor>Waldor, M., Davis, B. M. (2003). "Filamentous phages linked to virulence of Vibrio cholerae," <i>Current Opinion in Microbiology,Volume 6, Issue 1</i>, Pages 35-42, ISSN 1369-5274. https://doi.org/10.1016/S1369-5274(02)00005-X. (http://www.sciencedirect.com/science/article/pii/S136952740200005X)</ref><br> | ||
==Conclusion== | ==Conclusion== | ||
Revision as of 05:16, 12 December 2020
Overview
The CTXφ bacteriophage (or sometimes written as CTXphi bacteriophage) is a lysogenic, filamentous, single-stranded DNA (ssDNA) phage that is responsible for turning the previously non-infectious Vibrio cholerae into a highly pathogenic microbe that causes cholera in humans.[2][3][4] This particular bacteriophage,Vibrio virus CTXphi belongs to the family Inoviridae and the genus Affertcholeramvirus. These phages are composed of only 5 viral proteins and are between 1 and 2 microns in length.[5] They resemble something like a cooked spaghetti noodle. (See Figure 1 at right).
Genetic Material
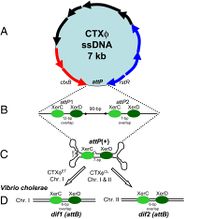
The CTXφ phage can be identified in its host in both its replicative form (when the bacteriophage is in lysogeny, or the lytic cycle), and in its non-replicative form, which is most common. It is in fact extremely rare to find or identify a CTXφ bacteriophage outside a host. This ssDNA phage, when in its lysogenic cycle, integrates its own genetic material in such a way as to form the most stable lysogens.[4] The average size of a CTXφ genome is about 6.9 to 7 kb long and consists of two sections; the RS2 region and the core region.[6] The core contains the genes that code for the Cholera toxin as well as proteins that are assumed to form the outside coat of the phage. The RS2 region is made up of genes that govern DNA synthesis and regulation as well as integration into host cells.[6]
Infection, Replication & Release from Host Cell
In a process known as lysogenic phage conversion, the CTXφ bacteriophage integrates, among others, its ctxAB genes into its host, Vibrio cholerae. The ctxAB genes code for a type of exotoxin, called Cholera toxin or just "CT," that causes V. cholerae to switch from being nonpathogenic to highly virulent. For those infected, it is also the primary cause of the large amounts of watered-down diarrhea—cholera's main symptom.[4] After finding a live host and undergoing CTXφ particle adsorption (see Figure 3 at right) to the V. cholerae cell wall, viral single-stranded DNA is then injected into the cell cytoplasm and a complementary strand of DNA is synthesized in order to form a circular plasmid, pCTX,[7]which then integrates into the V. cholerae genome at a site-specific attachment location on one or both chromosomes of V. cholerae.[4] Because the CTXφ phage, like all bacteriophages, only injects its genome, or, more commonly, its prophage, the CTXφ integration makes use of many of the host cell's enzymes. An integrase enzyme is not present in the CTXφ genome, so it recruits XerC and XerD, two tyrosine recombinases present in V. cholerae, to catalyze this merge.[4] Normally, XerC and XerD fix chromosome dimers in eubacteria.[8] CTXφ prophages usually integrate as tandem prophages,[9] getting replicated whenever the host cell's genome does. After sufficient time has passed and enough proteins and genetic material has been packaged, he phage proteins are localized to the inner membrane prior to forming virions.[9] Like many other filamentous phages, once emergence from the host cell occurs, it does not require lysis of the host's membrane. It is more of a secretion.[10] Secretion of CTXφ phages from the bacterial cell occurs through a host‐encoded channel, EpsD.[9]
CT & non-CT Toxins
The CTXφ phage contains toxins that are both harmful to human health and some that are essential for the functioning of the CTXφ phage itself.
CT Toxins
The epithelial cells of the small intestine contain a receptor called ganglioside GM1. This is a the natural biological receptor for cholera toxin.[11] Once infected with the CTXφ bacteriophage, Vibrio cholerae has the ability to potentially create new ganglioside GM1 receptors in the environment that the bacteria inhabits through an enzyme called sialidase. However, this doesn't seem to work well in humans, as a study in 1976 saw that no new GM1 receptors were created in the cells of intestinal epithelium.[11] Human intestines seem to have some sort of defenses put in place against enzymatic attacks like those from V. cholerae's sialidase.
Non-CT Toxins
Recent research suggests that there are perhaps two toxins (other than CT) that are produced from genes of the CTXφ genome. The first of these is accessory cholera enterotoxin, or Ace. Ace is currently thought to be a minor coat protein of CTXφ, though the process by which the toxin could be released from the protein coat is not yet known to scientists. The second non-CT toxin encoded within the CTXφ bacteriophage genome is zonula occludens toxin, or Zot. Zot, though absolutely essential for the production of the CTXφ virion, is not actually present in the phage particle. The part that Ace and Zot play in the virulence of cholera is still unknown.[7]
Conclusion
The CTXφ bacteriophage was a surprising discovery in 2001, especially when cholera is such an old disease; perhaps named in the 16th century and was around before then. Vibrio cholerae is quite harmless on its own, but when infected with the CTXφ phage, the host can become severely dehydrated and die without treatment. This disease is treatable with a class of antibiotics called tetracyclines, which are effective against gram-negative bacteria. Another option for treatment is hydration therapy. Cholera has been overshadowed by other diseases such as the zika virus and HIV/AIDS, but still remains an issue in third-world countries such as Africa and parts of Latin America.
References
- ↑ Gagic, D., Ciric M., Wen W., Ng F., Rakonjac J. (2016). "Exploring the Secretomes of Microbes and Microbial Communities Using Filamentous Phage Display." Frontiers in Microbiology, 7:429. https://doi.org/10.3389/fmicb.2016.00429.
- ↑ Davis, B. M., Kimsey, H. H., Chang, W., & Waldor, M. K. (1999). "The Vibrio cholerae O139 Calcutta bacteriophage CTXφ is infectious and encodes a novel repressor." Journal of Bacteriology, 181(21), 6779-6787. https://www.frontiersin.org/articles/10.3389/fmicb.2016.00429/full
- ↑ Ochman, H., Lawrence, J. & Groisman, E. (2000). "Lateral gene transfer and the nature of bacterial innovation." Nature, 405, 299–304. https://doi.org/10.1038/35012500.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Boyd, E. F. (2010). "Efficiency and specificity of CTXphi chromosomal integration: dif makes all the difference." Proceedings of the National Academy of Sciences of the United States of America, 107(9), 3951–3952. https://doi.org/10.1073/pnas.1000310107
- ↑ Mai-Prochnow, A., Hui, J., Kjelleberg, S., Rakonjac, J., McDougald, D., Rice, S. (2015). "Big things in small packages: the genetics of filamentous phage and effects on fitness of their host." FEMS Microbiology Reviews, Volume 39, Issue 4, Pages 465–487, https://doi.org/10.1093/femsre/fuu007
- ↑ 6.0 6.1 Fan, F., Kan, B.(2015). "Survival and proliferation of the lysogenic bacteriophage CTXφ in Vibrio cholerae." Virologica Sinica. 30, 19–25. https://doi.org/10.1007/s12250-014-3550-7
- ↑ 7.0 7.1 Waldor, M., Davis, B. M. (2003). "Filamentous phages linked to virulence of Vibrio cholerae," Current Opinion in Microbiology,Volume 6, Issue 1, Pages 35-42, ISSN 1369-5274. https://doi.org/10.1016/S1369-5274(02)00005-X. (http://www.sciencedirect.com/science/article/pii/S136952740200005X)
- ↑ Sherratt, D. J., Søballe, B., Barre, F. X., Filipe, S., Lau, I., Massey, T., & Yates, J. (2004). "Recombination and chromosome segregation." Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 359(1441), 61–69. https://doi.org/10.1098/rstb.2003.1365
- ↑ 9.0 9.1 9.2 McLeod, S. M., Kimsey, H. H., Davis, B. M., Waldor, M. K. (2005). "CTXφ and Vibrio cholerae: exploring a newly recognized type of phage–host cell relationship." Microreview, 57 (2), 347–356. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2958.2005.04676.x.
- ↑ Faruque, S. M., & Mekalanos, J. J. (2012). "Phage-bacterial interactions in the evolution of toxigenic Vibrio cholerae." Virulence, 3(7), 556–565. https://doi.org/10.4161/viru.22351
- ↑ 11.0 11.1 Holmgren, J. (1981). "Actions of cholera toxin and the prevention and treatment of cholera." Nature, 292(5822), 413-417.
Edited by Tara Cerny, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2019, Kenyon College.