Candida albicans (Pathogenesis): Difference between revisions
(Created page with "Category:Pages edited by students of Tyrrell Conway at the University of Oklahoma {{curated}} [[Image:OULOGOBIANCO.JPEG|thumb|230px|left|University of Oklahoma Study Abroa...") |
No edit summary |
||
| (47 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Conway}} | |||
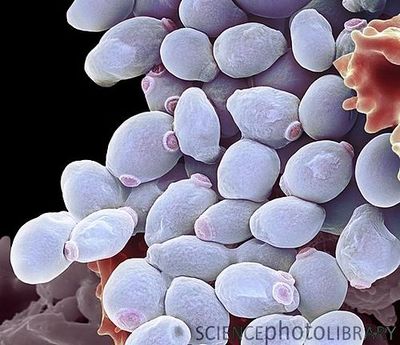
{{ | [[File:3D Candida albicans model.jpeg|400px|thumb|right|Scanning electron microscope image of <i>Candida albicans</i> yeast cells. From: Science Photo Library [http://33.media.tumblr.com/tumblr_m621kbaOaR1rv4l4do1_1280.jpg]]] | ||
[[File:3D | |||
==Etiology/Bacteriology== | ==Etiology/Bacteriology== | ||
===Taxonomy=== | ===Taxonomy=== | ||
<b>Domain</b> = [[Eukaryota]] <br> | |||
<b>Phylum</b> = [[Ascomycota]] <br> | |||
<b>Class</b> = [[Saccharomycetes]] <br> | |||
<b>Order</b> = [[Saccharomycetales]] <br> | |||
<b>Family</b> = [[Saccharomycetaceae]] <br> | |||
<b>Genus</b> = <i>Candida</i> <br> | |||
<b>species</b> = <i>albicans</i> <br> | |||
{| | {| | ||
| height="10" bgcolor="#FFDF95" | | | height="10" bgcolor="#FFDF95" | | ||
'''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode= | '''NCBI: [http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Tree&id=5476&lvl=3&lin=f&keep=1&srchmode=1&unlock Taxonomy] Genome: <font size="2">[http://www.ncbi.nlm.nih.gov/genome/?term=candida+albicans Candida albicans]</font>''' | ||
|} | |} | ||
===Description=== | ===Description=== | ||
<i> | |||
<i>Candida albicans</i> is an opportunistic fungal pathogen that is responsible for candidiasis in human hosts. <i>C. albicans</i> grow in several different morphological forms, ranging from unicellular budding yeast to true hyphae with parallel-side wall [[#References|[1]]]. Typically, <i>C. albicans</i>live as harmless commensals in the gastrointestinal and genitourinary tract and are found in over 70% of the population. Overgrowth of these organisms, however, will lead to disease, and it usually occurs in immunocompromised individuals, such as HIV-infected victims, transplant recipients, chemotherapy patients, and low birth-weight babies [[#References|[2]]]. There are three major forms of disease: oropharyngeal candidiasis, vulvovaginal candidiasis, and invasive candidiasis. Over 75% of women will suffer from a <i>C. albicans</i> infection, usually vulvovaginal candidiasis, in their lifetimes, and 40-50% of them will have additional occurrences(s). Interestingly, <i>C. albicans</i> are the 4th leading cause for nosocomial infections in patients’ bloodstreams. This could result in an extremely life-threatening, systemic infection in hospital patients with a mortality rate of 30% [[#References|[3]]]. For oropharyngeal candidiasis, infection occurs in the mouth or throat, and is identified by white plaque growth on oral mucous membranes. Vulvovaginal candidiasis or a “yeast infection” is the overgrowth of <i>C. albicans</i> in the vagina, and results in rash, itchiness, and discharge from the genital region. Lastly, invasive candidiasis occurs when the fungal pathogen enters the bloodstream and can easily spread to organs throughout the body. Invasive candidiasis is best identified when antibiotics fail to cure a patient’s fever [[#References|[4]]]. <i>C. albicans</i> infections are usually treatable with fluconazole, while severe infections require amphotericin B. | |||
==Pathogenesis== | ==Pathogenesis== | ||
===Transmission=== | ===Transmission=== | ||
<i>Candida albicans</i> is usually transmitted from mother to infant through childbirth, and remains as part of a normal human’s microflora. The overgrowth of <i>C. albicans</i> leads to symptoms of disease, and it occurs when there are imbalances – for example, changes in the normal acidity of the vagina. <i>C. albicans</i> infections very rarely spread through sexual intercourse. The typical reservoir for <i>C. albicans</i> is in the normal human microflora, and is not found in animal vectors [[#References|[5]]]. People-to-people acquired infections mostly happen in hospital settings where immunocompromised patients acquire the yeast from healthcare workers; studies show about a 40% incident rate [[#References|[6]]]. | |||
===Infectious dose, incubation, colonization=== | |||
There is no exact known infectious dose of <i>Candida albicans</i>. This is mostly due to the fact that a <i>C. albicans</i> infection stems from the commensal population of <i>C. albicans</i> in the human microflora. Candidiasis is caused by the abnormal growth in <i>C. albicans</i>, which is usually due to an imbalance in the environment. Usually, this imbalance occurs in a woman’s vagina – this infection less likely to occur for men. Several events can spark an imbalance. For example, antibiotic use can decrease the amount of lactobacillus bacteria, which decreases the amount of acidic products and the pH of the vagina. Other events are pregnancy, uncontrolled diabetes, impaired immune system, and irritation of the vagina. <i>C. albicans</i> are able to take advantage of the conditions and outcompete the normal microflora, resulting in candidiasis or a yeast infection [[#References|[7]]]. | |||
===Epidemiology=== | |||
=== | ====United States==== | ||
In the United States, oropharyngeal colonization by <i>Candida albicans</i> can be found in almost 30-55% of young adults. Also, the presence of <i>C. albicans</i> is detected in about 40-65% of normal fecal samples [[#References|[8]]]. Overall, <i>C. albicans</i> infections remain as the top source of fungal infections in immunocompromised people. For example, in HIV compromised patients, over 90% will develop a case of oropharyngeal candidiasis [[#References|[9]]]. On the other hand, about 75% of women experience vulvovaginal candidiasis, and about 40-50% will experience more than one episode. <br> | |||
[[ | |||
In the United States | In addition, candidemia is the fourth most common bloodstream infection in the United States. Almost 6.9 out of every 1000 intensive care unit patients are suffer from candidemia [[#References|[10]]]. <br> | ||
====Worldwide==== | ====Worldwide==== | ||
Rates for candidiasis and candidemia are similar throughout the world [[#References|[8]]]. | |||
===Virulence factors=== | ===Virulence factors=== | ||
==== | ====Polymorphism==== | ||
[[File:Morphology-2.jpg|400px|thumb|right| <b> Different Morphologies: </b> <br> A: Budding Yeast <br> B: Pseudohyphae <br> C: Hyphae <br> From: uvm.edu [http://www.uvm.edu/microbiology/Calbicanscells.jpg]]] | |||
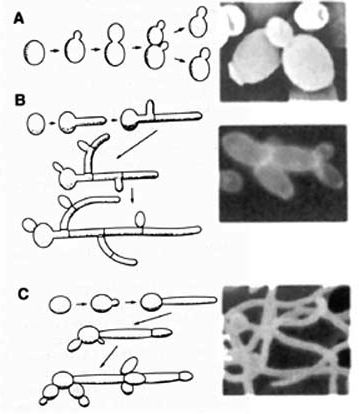
<i>Candida albicans</i> is a polymorphic fungus that can grow in several different forms, primarily yeast, pseudohyphae, and hyphae. For its pathogenicity, its ovoid-shaped budding yeast and parallel-walled true hyphae forms are the most important. The hyphae form is more prevalent for an infection, while the yeast form is believed to be important in the spread of <i>C. albicans</i>. The role of pseudohyphae is not very well understood, other than being an intermediate form between yeast and hyphae [[#References|[1]]]. Several factors can cause a change in morphology, such as pH differences, temperature changes, carbon dioxide levels, starvation, and quorum-sensing molecules (farnesol, tyrosol, and dodecanol) [[#References|[11]]]. | |||
==== | ====Adhesins==== | ||
<i> | <i>Candida albicans</i> have special sets of glycosylphosphatidylinositol (GPI)-linked cell surface glycoproteins that allow it to adhere to the surfaces of microorganisms. These glycoproteins are encoded by 8 sets of agglutinin-like sequence (ALS) genes, ranging from Als1-7 and Als9. For adhesion, the Als3 gene appears to the most important as it is upregulated during an infection of oral and vaginal epithelial cells. Also, it helps with biofilm formation by helping with adhesion to each other [[#References|[12]]]. | ||
==== | ====Invasins==== | ||
Along with adhesion, Als3 proteins can function as invasins that help with the invasion of <i>C. albicans</i> into host epithelial and endothelial cells. Another important invasin gene is Ssa1, which normally codes for heat-shock proteins. Basically, these specialized proteins on the pathogen’s surface mediate binding to host ligands, such as E-cadherin on epithelial cells and N-cadherin on endothelial cells, and it induces host cells to engulf the fungal pathogen. Another method of invasion is the active penetration of <i>C. albicans</i> into host cells by an unknown mechanism involving hyphae [[#References|[13]]]. | |||
==== | ====Biofilm formation==== | ||
<i>Candida albicans</i> have the ability to form biofilms on living and non-living surfaces, such as mucosal membranes and catheters, respectively. After the adherence of yeast cells to the surface, there is development of hyphae cells in the upper part of the biofilm. Eventually, this leads to a more resistant, mature biofilm and the dispersion of yeast cells – both contributing to the pathogen’s virulence. In the process of biofilm formation, Bcr1, Tec1 and Efg1 function as important transcriptional factors [[#References|[14]]]. Recent studies show that biofilms protect <i>C. albicans</i> colonization from neutrophil attack and deter the formation of reactive oxygen species [[#References|[15]]]. | |||
====Secreted hydrolases==== | |||
<i>Candida albicans</i> secrete 3 main classes of hydrolases: proteases, phospholipases and lipases. It is proposed that these hydrolases help facilitate the pathogen’s active penetration into host cells and the uptake of extracellular nutrients from the environment. There are about 10 known secreted aspartic proteases (Sap1-10), and their exact contribution to pathogenicity is controversial. For phospholipases, there are 4 major classes (A, B, C, and D), and all 5 members of the B class are involved with the disruption of a host cell surface. Thirdly, lipases are consisted of 10 members (LIP1-10), and studies show that there is decreased virulence in their absent [[#References|[16]]]. | |||
====Metabolic adaption==== | |||
<i>Candida albicans</i> are usually found in the gastrointestinal microbiome of healthy individuals, and in this environment, nutrient levels are relatively high. However, during niche changes in the course of an infection, available nutrient levels will also change. Consequently, the fungus can quickly undergo metabolic adaption, such as their glycolysis, gluconeogenesis, and starvation responses [[#References|[17]]]. For example, in the case of candidemia, <i>C. albicans</i> infect the bloodstream, which is typically rich in glucose. Nevertheless, it might be phagocytosed into a macrophage or neutrophil, where it’s surrounded by ROS, RNS, and AMPs. In response, <i>C. albicans</i> quickly switch from its glycolysis to starvation response with the activation of the glyoxylate cycle. Due to this flexibility, <i>C. albicans</i> can infect almost every organ in a human host through the bloodstream, providing candidemia’s higher mortality rate. | |||
==Clinical features== | ==Clinical features== | ||
There are 3 major type of infections caused by Candida albicans: oropharyngeal candidiasis, vulvovaginal (genital) candidiasis, and invasive candidiasis (candidemia). | |||
===Symptoms=== | ===Symptoms=== | ||
=== | ====Oropharyngeal candidiasis==== | ||
[[File:Candidiasis.jpg|400px|thumb|right| Oropharyngeal Candidiasis: Thrush From: WebMD [http://img.webmd.boots.com/dtmcms/live/webmd_uk/consumer_assets/site_images/anatomy_pages/PRinc_photo_of_thrush_on_tongue.jpg]]] | |||
Oropharyngeal candidiasis is an infection in the mouth and throat area. Usually, it is characterized by the formation of white patches on top of the tongue and throughout the mouth, which is also known as “thrush”. Thrush can be removed with a blade or a cotton-tipped swab, but the underlying tissue will be irritable and show a distinct redness. This infected area will cause soreness and difficultly during eating [[#References|[18]]]. | |||
====Vulvovaginal (genital) candidiasis==== | |||
Vulvovaginal candidiasis is the infection of the genital region, typically the vaginal walls, in women. The vaginal yeast infection causes itchiness and a burning-sensation in the vagina and surrounding tissues. Also, a white discharge – described with an appearance similar to white cottage cheese – is typically present. Genital candidiasis is much more prevalent in women, but men can also contract it. Although it is not considered an STD, men are usually infected after sex with a woman having a vaginal yeast infection. Symptoms involved rash, irritation on the head and surrounding skin of the penis [[#References|[18]]]. | |||
====Invasive candidiasis (candidemia)==== | |||
Invasive candidiasis (or candidemia) is the infection of <i>C. albicans</i> into the bloodstream. This leads to its invasion of organs throughout the body, such as the kidney, liver, brain, and many more. Patients began to suffer from fevers, chills, fatigue, muscles aches, and abdominal pains. Typically, patients with compromised immune systems are only at risk, while healthy people are susceptible to oral/genital candidiasis. Compromised immune systems can be caused by chemotherapy, transplantation, broad-spectrum antibiotics, and much more [[#References|[19]]]. | |||
===Mortality=== | |||
Most patients can recover from oral and genital candidiasis after a treatment with antifungal such as fluconazole. On the other hand, candidemia is much more life-threatening infection. In one study, the mortality rate for patients with candidemia was about 34% [[#References|[20]]]. Shockingly, this figure almost doubled when treatment is delayed; i.e., the mortality rate was 78% when therapy was delayed for more than 48 hours [[#References|[21]]]. | |||
==== | ==Diagnosis== | ||
[[File:Microscope.jpg|400px|thumb|right| Patient sample of oral epithelial cells with <i>Candida albicans</i> yeast and hyphae form cells <br> From: dartmouth.edu [http://www.dartmouth.edu/~sundstrom/images/image2.jpg]]] | |||
<i> | |||
<br> | |||
Oral and genital candidiasis are diagnosed in similar manners. After recognizing the rash, healthcare providers usually scrape at the affected area, and the sample is studied under a microscope; vaginal secretions can also be used as samples. For a positive result, there is typically an abundance of <i>Candida albicans</i> microorganisms. Fungal cultures are avoided because <i>C. albicans</i> are normal inhabitants of the human body [[#References|[4]]]. | |||
Candidemia is primarily diagnosed through blood cultures; however, in many cases, it becomes the obvious infection when antibiotics fail to succeed. Currently, there are studies on the use of noninvasive biomarkers, which include the serological markers: mannan, antimannan and (1,3)-β-d-glucan. Early clinical testing has noted the success of (1,3)-β-d-glucan assay for the early diagnostic of candidemia in ICU patients. Nevertheless, the problems with the assay involve its high cost and frequency of false-positives [[#References|[10]]]. | |||
=== | ==Treatment== | ||
In the event of candidiasis, the primary treatment for healthy adults is fluconazole (a triazole) with 800 mg loading dose, then 400 mg daily. For neutropenic patients, echinocandin (caspofungin, micafungin, or anidulafungin) or amphotericin B is preferred [[#References|[22]]]. Candidemia patients are usually administered fluconazole through IV, but for critically-ill patients, echinocandin and lipid formulation amphotericin B are again preferred. Also, studies show that treatments with a low-dose versus a high-dose of amphotericin B resulted with 40% less side-effects, and both treatments had the same effect on clearing the infection. Nevertheless, treatment with fluconazole resulted in the least side effects [[#References|[20]]]. | |||
==Prevention== | |||
Candidiasis is mainly caused by overgrowth of the <i>Candida albicans</i>. Keeping a healthy lifestyle is one of the main keys in protecting an individual from being burdened by the microorganism. Good hygiene, proper nutrition, and careful antibiotic use prevent <i>C. albicans</i> from outcompeting other commensal microorganisms. Immunocompromised individuals such as HIV, cancer, ICU, surgical, and transplant patients can experience recurrent infections or candidemia, but anti-fungal drugs, such as clotrimazole (Lotrimin, Mycelex), can help in their situation [[#References|[23]]]. | |||
==Host Immune Response== | |||
<i>Candida albicans</i> can successfully evade much of the immune system’s immunological surveillance, so it can commensally exists on mucosal surfaces. Studies have shown that the innate and adaptive immune systems play a role in the clearing of fungal growth. T Helper I cells produced cytokines that are important in activating phagocytes to a fungicidal state. On the contrary, T helper II cells appeared to be producing cytokines that were turning off the fungicidal effector capabilities. For cytokines, studies were showing that reduced production of IL-4 and IL-10 and increased production of IFN-γ and IL-2 helped mice resist infection. However, the complete absence of IL-4 and IL-10 was not advantageous either, but rather a finely regulated balance was the key. Also, studies have showed that neutrophils have an essential immunoregulatory role by releasing important cytokines, such as IL-10 and IL-12, to help antifungal T cell developement. This helps explain the fact that neutropenic patients have high risks for fungal infection. Lastly, <i>C. albicans</i> were able to elicit two different responses by dendritic cells when phagocytosed in yeast or hyphae form. Whenever yeast cells are phagocytosed, dendritic cells began a typical antifungal immune response, but hyphae cells are able to break out of the phagosome of dendritic cells [[#References|[24]]. | |||
==References== | |||
1. Sudbery P, Gow N, Berman J. 2004. The distinct morphogenic states of <i>Candida albicans</i>. Trends in Microbiology. 12(7):317-24. <br> | |||
2. Kabir MA, Hussain MA, Ahmad Z. 2012. <i>Candida albicans</i>: A Model Organism for Studying Fungal Pathogens. ISRN Microbiology. 2012: 538694. <br> | |||
3. Pfaller MA, Diekema DJ. 2007. Epidemiology of Invasive Candidiasis: a Persistent Public Health Problem. Virulence. (2): 119–128. <br> | |||
4. Centers for Disease Control and Prevention. Candidiasis. [<http://www.cdc.gov/fungal/diseases/candidiasis/index.html/>].<br> | |||
5. Public Health Agency of Canada. <i>Candida albicans</i> - Material Safety Data Sheets. [<http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/msds30e-eng.php>].<br> | |||
6. Fanelloa S, Boucharab JP, Jousseta N, Delbosa V, LeFlohicc AM. 2001. Nosocomial <i>Candida albicans</i> acquisition in a geriatric unit: epidemiology and evidence for person-to-person transmission. Journal of Hospital Infection. 47(1):46-52. <br> | |||
7. Mayo Clinic. Diseases and Conditions: Yeast infection (vaginal). [<http://www.mayoclinic.org/diseases-conditions/yeast-infection/basics/definition/con-20035129>].<br> | |||
8. Hidalgo JA, Vazquez JA, Bronze MS. 2014. Candidiasis: Frequency. [<http://emedicine.medscape.com/article/213853-overview#aw2aab6b2b3aa>].<br> | |||
9. de Repentigny L, Lewandowski D, Jolicoeur P. 2004. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clinical Microbiology Review. 17(4):729-59. <br> | |||
10. Mikulska M, Bono VD, Ratto S, Viscoli C. 2012. Occurrence, Presentation and Treatment of Candidemia. Expert Review of Clinical Immunology. 8(8):755-765. <br> | |||
11. Mayer FL, Wilson D, Hube B. 2013. <i>Candida albicans</i> pathogenicity mechanisms. Virulence. 4(2): 119–128. <br> | |||
12. Murciano C, Moyes DL, Runglall M, Tobouti P, Islam A, Hoyer LL, Naglik JR. 2012. Evaluation of the Role of <i>Candida albicans</i> Agglutinin-Like Sequence (Als) Proteins in Human Oral Epithelial Cell Interactions. PLoS One. 7(3): e33362. <br> | |||
13. Wächtler B, Wilson D, Haedicke K, Dalle F, Hube B. 2011. From Attachment to Damage: Defined Genes of <i>Candida albicans</i>Mediate Adhesion, Invasion and Damage during Interaction with Oral Epithelial Cells. PLoS One. 6(2): e17046. <br> | |||
14. Fanning S, Mitchell AP. 2012. Fungal Biofilms. PLoS Pathog. 8(4): e1002585. <br> | |||
15. Xie Z, Thompson A, Sobue T, Kashleva H, Xu H, Vasilakos J, Dongari-Bagtzoglou A. 2012. <i>Candida albicans</i> biofilms do not trigger reactive oxygen species and evade neutrophil killing. The Journal of Infectious Diseases. 206(12):1936-45. <br> | |||
16. Wächtler B, Citiulo F, Jablonowski N, Förster S, Dalle F, Schaller M. 2012. <i>Candida albicans</i>-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One. 7:e36952. <br> | |||
17. Brock M. 2009. Fungal metabolism in host niches. Current Opinion in Microbiology. 12(4):371-6. <br> | |||
18. eMedicineHealth. Candidiasis Yeast Infection Symptoms and Signs. [<http://www.emedicinehealth.com/candidiasis_yeast_infection/page3_em.htm#candidiasis_yeast_infection_symptoms_and_signs>].<br> | |||
19. American Thoracic Society. <i>Candida</i> Infection of the Bloodstream – Candidemia. [<http://patients.thoracic.org/information-series/en/resources/candidemia.pdf>]. <br> | |||
20. Nguyen MH, Peacock JE Jr, Tanner DC, Morris AJ, Nguyen ML, Snydman DR, Wagener MM, Yu VL. 1995. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Archives of Internal Medicine. 155(22):2429-35. <br> | |||
21. Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. 2002. Effects of nosocomial candidemia on outcomes of critically ill patients. American Journal of Medicine. 113(6):480-5. <br> | |||
22. Pappas PG, Kauffman CA, Andes D, Benjamin DK., Calandra TF, Edwards JE, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 48 (5): 503-535. <br> | |||
23. Prevention. Candidiasis. [<http://www.prevention.com/health-conditions/candidiasis#Prevention>]. <br> | |||
24. Romani L. 2000. Innate and adaptive immunity in <i>Candida albicans</i> infections and saprophytism. Journal of Leukocyte Biology. 68(2): 175-179. | |||
Created by | Created by Johnson Ong, a student of Tyrrell Conway at the University of Oklahoma. | ||
Latest revision as of 14:19, 11 February 2016

Etiology/Bacteriology
Taxonomy
Domain = Eukaryota
Phylum = Ascomycota
Class = Saccharomycetes
Order = Saccharomycetales
Family = Saccharomycetaceae
Genus = Candida
species = albicans
|
NCBI: Taxonomy Genome: Candida albicans |
Description
Candida albicans is an opportunistic fungal pathogen that is responsible for candidiasis in human hosts. C. albicans grow in several different morphological forms, ranging from unicellular budding yeast to true hyphae with parallel-side wall [1]. Typically, C. albicanslive as harmless commensals in the gastrointestinal and genitourinary tract and are found in over 70% of the population. Overgrowth of these organisms, however, will lead to disease, and it usually occurs in immunocompromised individuals, such as HIV-infected victims, transplant recipients, chemotherapy patients, and low birth-weight babies [2]. There are three major forms of disease: oropharyngeal candidiasis, vulvovaginal candidiasis, and invasive candidiasis. Over 75% of women will suffer from a C. albicans infection, usually vulvovaginal candidiasis, in their lifetimes, and 40-50% of them will have additional occurrences(s). Interestingly, C. albicans are the 4th leading cause for nosocomial infections in patients’ bloodstreams. This could result in an extremely life-threatening, systemic infection in hospital patients with a mortality rate of 30% [3]. For oropharyngeal candidiasis, infection occurs in the mouth or throat, and is identified by white plaque growth on oral mucous membranes. Vulvovaginal candidiasis or a “yeast infection” is the overgrowth of C. albicans in the vagina, and results in rash, itchiness, and discharge from the genital region. Lastly, invasive candidiasis occurs when the fungal pathogen enters the bloodstream and can easily spread to organs throughout the body. Invasive candidiasis is best identified when antibiotics fail to cure a patient’s fever [4]. C. albicans infections are usually treatable with fluconazole, while severe infections require amphotericin B.
Pathogenesis
Transmission
Candida albicans is usually transmitted from mother to infant through childbirth, and remains as part of a normal human’s microflora. The overgrowth of C. albicans leads to symptoms of disease, and it occurs when there are imbalances – for example, changes in the normal acidity of the vagina. C. albicans infections very rarely spread through sexual intercourse. The typical reservoir for C. albicans is in the normal human microflora, and is not found in animal vectors [5]. People-to-people acquired infections mostly happen in hospital settings where immunocompromised patients acquire the yeast from healthcare workers; studies show about a 40% incident rate [6].
Infectious dose, incubation, colonization
There is no exact known infectious dose of Candida albicans. This is mostly due to the fact that a C. albicans infection stems from the commensal population of C. albicans in the human microflora. Candidiasis is caused by the abnormal growth in C. albicans, which is usually due to an imbalance in the environment. Usually, this imbalance occurs in a woman’s vagina – this infection less likely to occur for men. Several events can spark an imbalance. For example, antibiotic use can decrease the amount of lactobacillus bacteria, which decreases the amount of acidic products and the pH of the vagina. Other events are pregnancy, uncontrolled diabetes, impaired immune system, and irritation of the vagina. C. albicans are able to take advantage of the conditions and outcompete the normal microflora, resulting in candidiasis or a yeast infection [7].
Epidemiology
United States
In the United States, oropharyngeal colonization by Candida albicans can be found in almost 30-55% of young adults. Also, the presence of C. albicans is detected in about 40-65% of normal fecal samples [8]. Overall, C. albicans infections remain as the top source of fungal infections in immunocompromised people. For example, in HIV compromised patients, over 90% will develop a case of oropharyngeal candidiasis [9]. On the other hand, about 75% of women experience vulvovaginal candidiasis, and about 40-50% will experience more than one episode.
In addition, candidemia is the fourth most common bloodstream infection in the United States. Almost 6.9 out of every 1000 intensive care unit patients are suffer from candidemia [10].
Worldwide
Rates for candidiasis and candidemia are similar throughout the world [8].
Virulence factors
Polymorphism
Candida albicans is a polymorphic fungus that can grow in several different forms, primarily yeast, pseudohyphae, and hyphae. For its pathogenicity, its ovoid-shaped budding yeast and parallel-walled true hyphae forms are the most important. The hyphae form is more prevalent for an infection, while the yeast form is believed to be important in the spread of C. albicans. The role of pseudohyphae is not very well understood, other than being an intermediate form between yeast and hyphae [1]. Several factors can cause a change in morphology, such as pH differences, temperature changes, carbon dioxide levels, starvation, and quorum-sensing molecules (farnesol, tyrosol, and dodecanol) [11].
Adhesins
Candida albicans have special sets of glycosylphosphatidylinositol (GPI)-linked cell surface glycoproteins that allow it to adhere to the surfaces of microorganisms. These glycoproteins are encoded by 8 sets of agglutinin-like sequence (ALS) genes, ranging from Als1-7 and Als9. For adhesion, the Als3 gene appears to the most important as it is upregulated during an infection of oral and vaginal epithelial cells. Also, it helps with biofilm formation by helping with adhesion to each other [12].
Invasins
Along with adhesion, Als3 proteins can function as invasins that help with the invasion of C. albicans into host epithelial and endothelial cells. Another important invasin gene is Ssa1, which normally codes for heat-shock proteins. Basically, these specialized proteins on the pathogen’s surface mediate binding to host ligands, such as E-cadherin on epithelial cells and N-cadherin on endothelial cells, and it induces host cells to engulf the fungal pathogen. Another method of invasion is the active penetration of C. albicans into host cells by an unknown mechanism involving hyphae [13].
Biofilm formation
Candida albicans have the ability to form biofilms on living and non-living surfaces, such as mucosal membranes and catheters, respectively. After the adherence of yeast cells to the surface, there is development of hyphae cells in the upper part of the biofilm. Eventually, this leads to a more resistant, mature biofilm and the dispersion of yeast cells – both contributing to the pathogen’s virulence. In the process of biofilm formation, Bcr1, Tec1 and Efg1 function as important transcriptional factors [14]. Recent studies show that biofilms protect C. albicans colonization from neutrophil attack and deter the formation of reactive oxygen species [15].
Secreted hydrolases
Candida albicans secrete 3 main classes of hydrolases: proteases, phospholipases and lipases. It is proposed that these hydrolases help facilitate the pathogen’s active penetration into host cells and the uptake of extracellular nutrients from the environment. There are about 10 known secreted aspartic proteases (Sap1-10), and their exact contribution to pathogenicity is controversial. For phospholipases, there are 4 major classes (A, B, C, and D), and all 5 members of the B class are involved with the disruption of a host cell surface. Thirdly, lipases are consisted of 10 members (LIP1-10), and studies show that there is decreased virulence in their absent [16].
Metabolic adaption
Candida albicans are usually found in the gastrointestinal microbiome of healthy individuals, and in this environment, nutrient levels are relatively high. However, during niche changes in the course of an infection, available nutrient levels will also change. Consequently, the fungus can quickly undergo metabolic adaption, such as their glycolysis, gluconeogenesis, and starvation responses [17]. For example, in the case of candidemia, C. albicans infect the bloodstream, which is typically rich in glucose. Nevertheless, it might be phagocytosed into a macrophage or neutrophil, where it’s surrounded by ROS, RNS, and AMPs. In response, C. albicans quickly switch from its glycolysis to starvation response with the activation of the glyoxylate cycle. Due to this flexibility, C. albicans can infect almost every organ in a human host through the bloodstream, providing candidemia’s higher mortality rate.
Clinical features
There are 3 major type of infections caused by Candida albicans: oropharyngeal candidiasis, vulvovaginal (genital) candidiasis, and invasive candidiasis (candidemia).
Symptoms
Oropharyngeal candidiasis
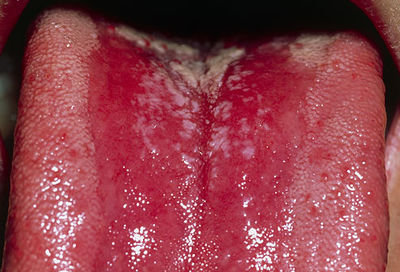
Oropharyngeal candidiasis is an infection in the mouth and throat area. Usually, it is characterized by the formation of white patches on top of the tongue and throughout the mouth, which is also known as “thrush”. Thrush can be removed with a blade or a cotton-tipped swab, but the underlying tissue will be irritable and show a distinct redness. This infected area will cause soreness and difficultly during eating [18].
Vulvovaginal (genital) candidiasis
Vulvovaginal candidiasis is the infection of the genital region, typically the vaginal walls, in women. The vaginal yeast infection causes itchiness and a burning-sensation in the vagina and surrounding tissues. Also, a white discharge – described with an appearance similar to white cottage cheese – is typically present. Genital candidiasis is much more prevalent in women, but men can also contract it. Although it is not considered an STD, men are usually infected after sex with a woman having a vaginal yeast infection. Symptoms involved rash, irritation on the head and surrounding skin of the penis [18].
Invasive candidiasis (candidemia)
Invasive candidiasis (or candidemia) is the infection of C. albicans into the bloodstream. This leads to its invasion of organs throughout the body, such as the kidney, liver, brain, and many more. Patients began to suffer from fevers, chills, fatigue, muscles aches, and abdominal pains. Typically, patients with compromised immune systems are only at risk, while healthy people are susceptible to oral/genital candidiasis. Compromised immune systems can be caused by chemotherapy, transplantation, broad-spectrum antibiotics, and much more [19].
Mortality
Most patients can recover from oral and genital candidiasis after a treatment with antifungal such as fluconazole. On the other hand, candidemia is much more life-threatening infection. In one study, the mortality rate for patients with candidemia was about 34% [20]. Shockingly, this figure almost doubled when treatment is delayed; i.e., the mortality rate was 78% when therapy was delayed for more than 48 hours [21].
Diagnosis
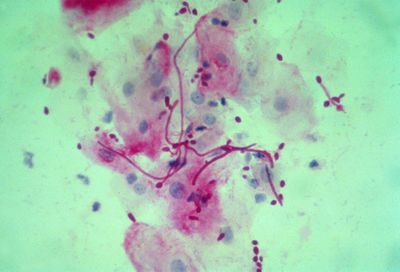
From: dartmouth.edu [5]
Oral and genital candidiasis are diagnosed in similar manners. After recognizing the rash, healthcare providers usually scrape at the affected area, and the sample is studied under a microscope; vaginal secretions can also be used as samples. For a positive result, there is typically an abundance of Candida albicans microorganisms. Fungal cultures are avoided because C. albicans are normal inhabitants of the human body [4].
Candidemia is primarily diagnosed through blood cultures; however, in many cases, it becomes the obvious infection when antibiotics fail to succeed. Currently, there are studies on the use of noninvasive biomarkers, which include the serological markers: mannan, antimannan and (1,3)-β-d-glucan. Early clinical testing has noted the success of (1,3)-β-d-glucan assay for the early diagnostic of candidemia in ICU patients. Nevertheless, the problems with the assay involve its high cost and frequency of false-positives [10].
Treatment
In the event of candidiasis, the primary treatment for healthy adults is fluconazole (a triazole) with 800 mg loading dose, then 400 mg daily. For neutropenic patients, echinocandin (caspofungin, micafungin, or anidulafungin) or amphotericin B is preferred [22]. Candidemia patients are usually administered fluconazole through IV, but for critically-ill patients, echinocandin and lipid formulation amphotericin B are again preferred. Also, studies show that treatments with a low-dose versus a high-dose of amphotericin B resulted with 40% less side-effects, and both treatments had the same effect on clearing the infection. Nevertheless, treatment with fluconazole resulted in the least side effects [20].
Prevention
Candidiasis is mainly caused by overgrowth of the Candida albicans. Keeping a healthy lifestyle is one of the main keys in protecting an individual from being burdened by the microorganism. Good hygiene, proper nutrition, and careful antibiotic use prevent C. albicans from outcompeting other commensal microorganisms. Immunocompromised individuals such as HIV, cancer, ICU, surgical, and transplant patients can experience recurrent infections or candidemia, but anti-fungal drugs, such as clotrimazole (Lotrimin, Mycelex), can help in their situation [23].
Host Immune Response
Candida albicans can successfully evade much of the immune system’s immunological surveillance, so it can commensally exists on mucosal surfaces. Studies have shown that the innate and adaptive immune systems play a role in the clearing of fungal growth. T Helper I cells produced cytokines that are important in activating phagocytes to a fungicidal state. On the contrary, T helper II cells appeared to be producing cytokines that were turning off the fungicidal effector capabilities. For cytokines, studies were showing that reduced production of IL-4 and IL-10 and increased production of IFN-γ and IL-2 helped mice resist infection. However, the complete absence of IL-4 and IL-10 was not advantageous either, but rather a finely regulated balance was the key. Also, studies have showed that neutrophils have an essential immunoregulatory role by releasing important cytokines, such as IL-10 and IL-12, to help antifungal T cell developement. This helps explain the fact that neutropenic patients have high risks for fungal infection. Lastly, C. albicans were able to elicit two different responses by dendritic cells when phagocytosed in yeast or hyphae form. Whenever yeast cells are phagocytosed, dendritic cells began a typical antifungal immune response, but hyphae cells are able to break out of the phagosome of dendritic cells [24.
References
1. Sudbery P, Gow N, Berman J. 2004. The distinct morphogenic states of Candida albicans. Trends in Microbiology. 12(7):317-24.
2. Kabir MA, Hussain MA, Ahmad Z. 2012. Candida albicans: A Model Organism for Studying Fungal Pathogens. ISRN Microbiology. 2012: 538694.
3. Pfaller MA, Diekema DJ. 2007. Epidemiology of Invasive Candidiasis: a Persistent Public Health Problem. Virulence. (2): 119–128.
4. Centers for Disease Control and Prevention. Candidiasis. [<http://www.cdc.gov/fungal/diseases/candidiasis/index.html/>].
5. Public Health Agency of Canada. Candida albicans - Material Safety Data Sheets. [<http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/msds30e-eng.php>].
6. Fanelloa S, Boucharab JP, Jousseta N, Delbosa V, LeFlohicc AM. 2001. Nosocomial Candida albicans acquisition in a geriatric unit: epidemiology and evidence for person-to-person transmission. Journal of Hospital Infection. 47(1):46-52.
7. Mayo Clinic. Diseases and Conditions: Yeast infection (vaginal). [<http://www.mayoclinic.org/diseases-conditions/yeast-infection/basics/definition/con-20035129>].
8. Hidalgo JA, Vazquez JA, Bronze MS. 2014. Candidiasis: Frequency. [<http://emedicine.medscape.com/article/213853-overview#aw2aab6b2b3aa>].
9. de Repentigny L, Lewandowski D, Jolicoeur P. 2004. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clinical Microbiology Review. 17(4):729-59.
10. Mikulska M, Bono VD, Ratto S, Viscoli C. 2012. Occurrence, Presentation and Treatment of Candidemia. Expert Review of Clinical Immunology. 8(8):755-765.
11. Mayer FL, Wilson D, Hube B. 2013. Candida albicans pathogenicity mechanisms. Virulence. 4(2): 119–128.
12. Murciano C, Moyes DL, Runglall M, Tobouti P, Islam A, Hoyer LL, Naglik JR. 2012. Evaluation of the Role of Candida albicans Agglutinin-Like Sequence (Als) Proteins in Human Oral Epithelial Cell Interactions. PLoS One. 7(3): e33362.
13. Wächtler B, Wilson D, Haedicke K, Dalle F, Hube B. 2011. From Attachment to Damage: Defined Genes of Candida albicansMediate Adhesion, Invasion and Damage during Interaction with Oral Epithelial Cells. PLoS One. 6(2): e17046.
14. Fanning S, Mitchell AP. 2012. Fungal Biofilms. PLoS Pathog. 8(4): e1002585.
15. Xie Z, Thompson A, Sobue T, Kashleva H, Xu H, Vasilakos J, Dongari-Bagtzoglou A. 2012. Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing. The Journal of Infectious Diseases. 206(12):1936-45.
16. Wächtler B, Citiulo F, Jablonowski N, Förster S, Dalle F, Schaller M. 2012. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One. 7:e36952.
17. Brock M. 2009. Fungal metabolism in host niches. Current Opinion in Microbiology. 12(4):371-6.
18. eMedicineHealth. Candidiasis Yeast Infection Symptoms and Signs. [<http://www.emedicinehealth.com/candidiasis_yeast_infection/page3_em.htm#candidiasis_yeast_infection_symptoms_and_signs>].
19. American Thoracic Society. Candida Infection of the Bloodstream – Candidemia. [<http://patients.thoracic.org/information-series/en/resources/candidemia.pdf>].
20. Nguyen MH, Peacock JE Jr, Tanner DC, Morris AJ, Nguyen ML, Snydman DR, Wagener MM, Yu VL. 1995. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Archives of Internal Medicine. 155(22):2429-35.
21. Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. 2002. Effects of nosocomial candidemia on outcomes of critically ill patients. American Journal of Medicine. 113(6):480-5.
22. Pappas PG, Kauffman CA, Andes D, Benjamin DK., Calandra TF, Edwards JE, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 48 (5): 503-535.
23. Prevention. Candidiasis. [<http://www.prevention.com/health-conditions/candidiasis#Prevention>].
24. Romani L. 2000. Innate and adaptive immunity in Candida albicans infections and saprophytism. Journal of Leukocyte Biology. 68(2): 175-179.
Created by Johnson Ong, a student of Tyrrell Conway at the University of Oklahoma.