Central Metabolism (Flooded soils): Difference between revisions
| (45 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | {{Curated}} | ||
[[Image:FloodedSoil.png|600px|thumb|right|Comparison of water levels in three environments: unsaturated soil, saturated soil, and flooded soil. [http://www.floodsite.net/juniorfloodsite/html/en/student/thingstoknow/hydrology/waterstorage2.html Source]]] | [[Image:FloodedSoil.png|600px|thumb|right|Comparison of water levels in three environments: unsaturated soil, saturated soil, and flooded soil. [http://www.floodsite.net/juniorfloodsite/html/en/student/thingstoknow/hydrology/waterstorage2.html Source]]] | ||
[[Image: | [[Image:Peatland.jpg|thumb|500px|right|A peatland, a type of flooded environment with a layer of organic matter, in Australia. Peatlands produce a high amount of methane emissions. [http://photography.nationalgeographic.com/photography/photo-of-the-day/peatland-australia-essick/ Source]]] | ||
Flooded soils are a condition in which soil is oversaturated with water, often due to natural occurrence or with intended purpose for agricultural reasons. Perpetually flooded soils can be found in wetlands, swamps and marshes; temporary flooded soils can be an effect of season weather or agricultural practices. As water levels fluctuate, and soils alternate between flooded and unflooded states, the chemical makeup of the [https://microbewiki.kenyon.edu/index.php/Soil_Environment soil environment] will continuously change. A soil’s water content directly influences both inorganic and microbial reactions that affect the soil’s redox potential (E<sub>h</sub>), acidity, alkalinity, and salinity.<ref name="Campos"> | Flooded soils are a condition in which an area of soil is oversaturated with water, often due to natural occurrence or with intended purpose for agricultural reasons. Perpetually flooded soils can be found in wetlands, swamps and marshes; temporary flooded soils can be an effect of season weather or agricultural practices. As water levels fluctuate, and soils alternate between flooded and unflooded states, the chemical makeup of the [https://microbewiki.kenyon.edu/index.php/Soil_Environment soil environment] will continuously change. A soil’s water content directly influences both inorganic and microbial reactions that affect the soil’s redox potential (E<sub>h</sub>), acidity, alkalinity, and salinity.<ref name="Campos">Dassonville, F., & Renault, P. (2002). Interactions between microbial processes and geochemical transformations under anaerobic conditions: A review. Agronomie, 22(1), 51-68. Retrieved from http://www.agronomy-journal.org/articles/agro/abs/2002/01/05/05.html. </ref> | ||
One of the most important effects of | One of the most important effects of flooded soils is that the presence of oxygen is limited in such an environment, and any remaining oxygen is quickly used up via aerobic respiration. As a result, other compounds are used as electron acceptors in energy acquisition reactions; the microorganisms that specialize in conducting these other reactions are able to flourish and affect nutrient cycling in the ecosystem. | ||
Microbial transformations of elements in anaerobic soils play a worldwide role in biogeochemical cycling of nutrients and in greenhouse gas emissions. Changes in the oxidation state of terminal electron acceptors may result in nutrient loss from the system via volatilization or leaching. Anaerobic microbial processes including denitrification, methanogenesis, and methanotrophy are responsible for releasing greenhouse gases (N<sub>2</sub>O, CH<sub>4</sub>, CO<sub>2</sub>) into the atmosphere. <ref>United States Environmental Protection Agency. (n.d.). Overview of Greenhouse Gases. Retrieved March 13, 2016, from http://www3.epa.gov/climatechange/ghgemissions/gases/ch4.html .</ref> | Microbial transformations of elements in anaerobic soils play a worldwide role in biogeochemical cycling of nutrients and in greenhouse gas emissions. Changes in the oxidation state of terminal electron acceptors may result in nutrient loss from the system via volatilization or leaching. Anaerobic microbial processes including denitrification, methanogenesis, and methanotrophy are responsible for releasing greenhouse gases (N<sub>2</sub>O, CH<sub>4</sub>, CO<sub>2</sub>) into the atmosphere. <ref>United States Environmental Protection Agency. (n.d.). Overview of Greenhouse Gases. Retrieved March 13, 2016, from http://www3.epa.gov/climatechange/ghgemissions/gases/ch4.html .</ref> | ||
| Line 12: | Line 12: | ||
==Key Microbial Processes== | ==Key Microbial Processes== | ||
===Microbial Respiration: [http://en.wikipedia.org/wiki/Redox Oxidation/Reduction | ===Microbial Respiration: [http://en.wikipedia.org/wiki/Redox Oxidation/Reduction Reactions]=== | ||
[[Image:RedoxReaction.jpg|300px|thumb|left|General process of a paired reduction and oxidation. The transfer of electrons from molecule A to B is shown. [https://online.science.psu.edu/biol011_sandbox_7239/node/7381 Source]]] | [[Image:RedoxReaction.jpg|300px|thumb|left|General process of a paired reduction and oxidation. The transfer of electrons from molecule A to B is shown. [https://online.science.psu.edu/biol011_sandbox_7239/node/7381 Source]]] | ||
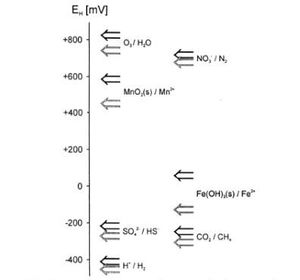
[[Image:Succession1. | [[Image:Succession1.JPG|300px|thumb|right|Redox potentials of various couples. In soil, the order of succession begins with oxygen and generally ends with carbon dioxide. <ref>Schüring, J., Schulz, H. D., & Fischer, W. R. (Eds.). (2000). Redox: Fundamentals, processes, and applications. New York City, NY: Springer.</ref>]] | ||
In order to obtain energy, many microbes make use of the process of respiration through | In order to obtain energy, many microbes make use of the process of respiration through an oxidation-reduction (redox) reaction. Respiration is a catabolic reaction that produces ATP in which either organic or inorganic compounds act as primary electron donors, and exogenous compounds act as the terminal electron acceptors. In a redox reaction, one molecule (the reducing agent) loses electrons and another molecule (the oxidizing agent) accepts electrons. Electron donors such as glucose, methanol, and [https://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide NADH] are energy sources that can be thought of as “giving up” their electrons, while another molecule is in need to receive said electrons. For example, in aerobic respiration, energy rich compounds like glucose (the reducing agent) are oxidized to carbon dioxide, with oxygen (the oxidizing agent) acting as a terminal electron acceptor and being reduced to water. In addition to oxygen, microorganisms use a large variety of electron acceptors. | ||
Depending on the type of electron acceptors used by microorganisms, microbes can be placed into a variety of classifications. Strict aerobes can only use oxygen as a terminal electron acceptor. Obligate anaerobes cannot use oxygen and are actually inhibited or poisoned by oxygen. Facultative anaerobes are flexible in electron acceptor usage; as a result of this they can make use of other redox reactions to maintain a supply of energy as oxygen levels decrease. | Depending on the type of electron acceptors used by microorganisms, microbes can be placed into a variety of classifications. Strict aerobes can only use oxygen as a terminal electron acceptor. Obligate anaerobes cannot use oxygen and are actually inhibited or poisoned by oxygen. Facultative anaerobes are flexible in electron acceptor usage; as a result of this they can make use of other redox reactions to maintain a supply of energy as oxygen levels decrease. | ||
Oxygen gas (O<sub>2</sub>) is one of the most favorable electron acceptors, but it is typically not available in flooded soils. Instead, facultative and strict anaerobic microbes utilize other oxidizing agents | Oxygen gas (O<sub>2</sub>) is one of the most favorable electron acceptors, but it is typically not available in flooded soils. Instead, facultative and strict anaerobic microbes utilize other oxidizing agents (electron acceptors) to carry out respiration. The amount of energy that can be obtained through respiration varies between compounds and microbes and will make use of these compounds in order of the decreasing redox potential, thus leading to a succession of acceptors. | ||
====Redox Potential (E<sub>h</sub>)==== | ====Redox Potential (E<sub>h</sub>)==== | ||
Redox potential is the tendency for a reaction, specifically the movement and transfer of electrons, to occur spontaneously and is reported as E<sub>h</sub> in mV. These measurements have been experimentally determined through aqueous solutions containing electrodes, one being the cathode (electron donator) and one being the anode (electron receiver). <ref>Redox Chemistry Primer. (n.d.). Retrieved March 12, 2016, from http://www.kgs.ku.edu/Hydro/GWtutor/Plume_Busters/remediate_refs/redox_chemistry.htm .</ref> | Redox potential is the tendency for a reaction, specifically the movement and transfer of electrons, to occur spontaneously and is reported as E<sub>h</sub> in mV. These measurements have been experimentally determined through aqueous solutions containing electrodes, one being the cathode (electron donator) and one being the anode (electron receiver). <ref>Redox Chemistry Primer. (n.d.). Retrieved March 12, 2016, from http://www.kgs.ku.edu/Hydro/GWtutor/Plume_Busters/remediate_refs/redox_chemistry.htm .</ref> | ||
| Line 30: | Line 29: | ||
===The Electron Tower=== | ===The Electron Tower=== | ||
[[Image:RedoxTower.jpg|300px|thumb|right|A tower showing common redox pairs. The greater the "distance" between a donor and acceptor, the greater the energy released. From ''Brock Biology of Microorganisms''.]] | [[Image:RedoxTower.jpg|300px|thumb|right|A tower showing common redox pairs. The greater the "distance" between a donor and acceptor, the greater the energy released. From ''Brock Biology of Microorganisms''.]] | ||
Microbes will successively use the highest energy yielding electron acceptors available in the order indicated on the electron tower, which is a ranking of common redox reactions by the amount of energy that can be obtained from them. Compounds are listed in redox pairs (oxidized form and reduced form) The greater the difference in electrical potential between the reactants and products of a reaction the the | Microbes will successively use the highest energy yielding electron acceptors available in the order indicated on the electron tower, which is a ranking of common redox reactions by the amount of energy that can be obtained from them. Compounds are listed in redox pairs (oxidized form and reduced form) The greater the difference in electrical potential between the reactants and products of a reaction, the greater the release of energy that is crucial for microbial growth. <ref>Madigan, M. T., Martinko, J. M., & Parker, J. (2003). Brock biology of microorganisms (10th ed.). Upper Saddle River, NJ: Prentice Hall/Pearson Education. </ref> | ||
O<sub>2</sub>, the lowest oxidizing agent on the tower, yields the most energy when reduced in a redox reaction with a specific electron donor and will be the first electron acceptor depleted when commonly available. In flooded soils, the amount of oxygen in the system will be very small. When the soil’s microbial population exhausts its remaining O<sub>2</sub>, it will begin using available electron acceptors which provide the next highest amount of energy. Competition generally limits the use of weaker electron acceptors; for example, iron reducers (using Fe<sup>3+</sup>) may exist in the soil but will be dominated by the presence of denitrifiers, who will growth faster with access to their stronger electron acceptor (NO<sub>3</sub><sup>-</sup>). Overall, this process of succession will continue as each electron acceptor supply is used. | O<sub>2</sub>, the lowest oxidizing agent on the tower, yields the most energy when reduced in a redox reaction with a specific electron donor and will be the first electron acceptor depleted when commonly available. In flooded soils, the amount of oxygen in the system will be very small. When the soil’s microbial population exhausts its remaining O<sub>2</sub>, it will begin using other available electron acceptors which provide the next highest amount of energy. Competition generally limits the use of weaker electron acceptors; for example, iron reducers (using Fe<sup>3+</sup>) may exist in the soil but will be dominated by the presence of denitrifiers, who will growth faster with access to their stronger electron acceptor (NO<sub>3</sub><sup>-</sup>). Overall, this process of succession will continue as each electron acceptor supply is used. | ||
===Succession of Electron Acceptors=== | ===Succession of Electron Acceptors=== | ||
The main succession of electron acceptor usage in flooded soils is as follows: | |||
Aerobic respiration: ½ O<sub>2</sub> + 2e<sup>-</sup> + 2H<sup>+</sup> -> H<sub>2</sub>O (by facultative anaerobes and aerobes) | |||
Denitrification: 2NO<sub>3</sub><sup>-</sup> + 12 H<sup>+</sup> +10e<sup>-</sup> -> N<sub>2</sub>+6H<sub>2</sub>O (by denitrifiers) | |||
Manganese reduction: MnO<sub>2</sub> + 4H<sup>+</sup> + 2e<sup>-</sup> ->Mn<sup>2+</sup> + 2H<sub>2</sub>O (by manganese reducing bacteria) | |||
Iron reduction: Fe(OH)<sub>3</sub> + 3 H<sup>+</sup> + 2e<sup>-</sup> -> Fe<sup>2+</sup> + 2H<sub>2</sub>O (by iron reducing bacteria) | |||
Sulfate reduction: SO<sub>4</sub><sup>2-</sup> + 10H<sup>+</sup> +8e<sup>-</sup> -> H<sub>2</sub>S + 4H<sub>2</sub>O (by sulfate reducing bacteria) | |||
Methane production: CO<sub>2</sub> + 8 H<sup>+</sup> + 8e<sup>-</sup> -> CH<sub>4</sub> +2 H<sub>2</sub>O (by methanogens) | |||
====[[Nitrogen Cycle|Nitrate Reduction]]==== | ====[[Nitrogen Cycle|Nitrate Reduction]]==== | ||
| Line 44: | Line 56: | ||
====Iron Reduction==== | ====Iron Reduction==== | ||
[[Image:pipe.jpg|100px|thumb|left|Corroded water main. [http://coloradogeologicalsurvey.org/geologic-hazards/corrosive-soils/corrosive-soil-damage/ Source] ]] | [[Image:pipe.jpg|100px|thumb|left|Corroded water main. [http://coloradogeologicalsurvey.org/geologic-hazards/corrosive-soils/corrosive-soil-damage/ Source] ]] | ||
The utilization of ferric iron ions (Fe<sup>3+</sup>), at approximately 120 mV, occurs when ions are released from metal deposits or minerals in the soil. Ferrous iron (Fe<sup>2+</sup>) product causes soil gleying, a process described | The utilization of ferric iron ions (Fe<sup>3+</sup>), at approximately 120 mV, occurs when ions are released from metal deposits or minerals in the soil. Ferrous iron (Fe<sup>2+</sup>) product causes soil gleying, (a process described in a later section) when it accumulates. The source of the iron is also of relevance; whereas iron reduction in phosphate minerals can release phosphate for other organisms, it can also lead to corrosion of steel where iron reducers are present. Some ''Pseudomonas'' species can release pseudobactin, an iron-binding compound that limits its availability to other bacteria. <ref name="Sylvia" /> | ||
====Sulfate Reduction==== | ====Sulfate Reduction==== | ||
Sulfate reduction begins occurring at 0 mV, and the dissimilatory reduction results in hydrogen sulfide (H<sub>2</sub>S) being released. However, H<sub>2</sub>S is prone to reaction with Fe<sup>2+</sub> to form iron sulfide (FeS). As a result, it often reacts before it reaches the surface of the soil, unable to disperse into the air. <ref name="Sylvia" /> Sulfate reduction has several documented consequences, ranging from corrosion of underground iron pipes due to FeS formation and blackening of soil caused by liberation of organic matter. Hydrogen sulfide is known as “swamp gas”, due to its emergence from one form of soil flooding, and has an odor comparable to rotting eggs. It | Sulfate reduction begins occurring at 0 mV, and the dissimilatory reduction results in hydrogen sulfide (H<sub>2</sub>S) being released. However, H<sub>2</sub>S is prone to reaction with Fe<sup>2+</sub> to form iron sulfide (FeS). As a result, it often reacts before it reaches the surface of the soil, unable to disperse into the air. <ref name="Sylvia" /> Sulfate reduction has several documented consequences, ranging from corrosion of underground iron pipes due to FeS formation and blackening of soil caused by liberation of organic matter. Hydrogen sulfide is known as “swamp gas”, due to its emergence from one form of soil flooding, and has an odor comparable to rotting eggs. It can accumulate in many bodies of water and the air above them; due to its flammability and toxicity, this is very dangerous. <ref>Occupational Safety & Health Administration. (n.d.). Safety and Health Topics | Hydrogen Sulfide. Retrieved February 23, 2016, from https://www.osha.gov/SLTC/hydrogensulfide/index.html .</ref> | ||
====Methanogenesis (by Carbon Dioxide Reduction)==== | ====Methanogenesis (by Carbon Dioxide Reduction)==== | ||
The process of using carbon dioxide (CO<sub>2</sub>) as a terminal electron acceptor results in the formation of methane (CH<sub>4</sub>) and is known as methanogenesis. In soil, | The process of using carbon dioxide (CO<sub>2</sub>) as a terminal electron acceptor results in the formation of methane (CH<sub>4</sub>) and is known as methanogenesis. In soil, methanogenesis occurs almost exclusively in a flooded condition due to its reduction potential being so low (below 100 mV). The use of CO<sub>2</sub> in this fashion yields much less energy than the reactions of previous electron acceptors, so this process has lower growth rates in turn. Organisms who perform methanogenesis are known as [[methanogens]] and are a group of anaerobic Archaea. CH<sub>4</sub> can also be produced as a result of acetate (CH<sub>3</sub>COOH) fermentation, which is also performed by methanogens. <ref name="Sylvia" /> | ||
===Fermentation in Flooded Soils=== | ===Fermentation in Flooded Soils (Non-Respiratory)=== | ||
Fermentation | Fermentation is a different form of metabolism from respiration that occurs in the absence of a suitable terminal electron acceptor. Cells convert NADH and pyruvate from the glycolysis of sugars into NAD+ and other compounds, depending on the species that is fermenting. The various products of fermentation, including alcohols, lactic acid, and acetate are released into the surrounding soil and then become available for use by other anaerobic organisms. Additionally, fermentation generally reduces soil pH, which will encourage dissolution of minerals and their subsequent access by bacteria. <ref name="Richardson">Richardson, J. L., & Vepraskas, M. J. (2001). Wetland soils: Genesis, hydrology, landscapes, and classification. Boca Raton, FL: Lewis. </ref> | ||
==Microorganisms Involved== | ==Microorganisms Involved== | ||
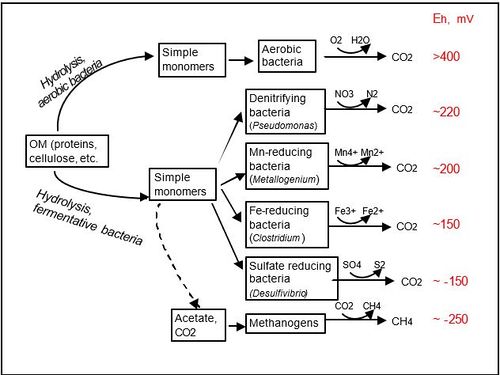
As available oxygen declines, organisms that thrive under anoxic conditions proliferate using alternative electron acceptors. The order in which available electron acceptors are consumed can generally be predicted by the electron tower and associated energy yields of electron pairs. Changes in redox conditions of flooded soils over time reflects the successive availability of terminal electron acceptors from the electron tower, and will govern which microbes will thrive through being able to use them. | |||
Some microbes below are able to oxidize the reduced form of their corresponding substance.<ref>Liesack, W. (2000). Microbiology of flooded rice paddies. FEMS Microbiology Reviews, 24(5), 625-645. </ref> | |||
[[Image:Processes.jpg|500px|thumb|right|Summary of reducing conditions in flooded soils. [http://www.des.ucdavis.edu/faculty/rejmankova/ESP155_Soils-2004.pdf Source]]] | |||
[[Image: | |||
{| width="800" border="1" | |||
|----- bgcolor ="grey" | |||
| width="200" height="25" | '''Process''' | |||
| width="1000" | '''Example Genera of Common Bacteria Involved''' | |||
{| width=" | |||
|----- bgcolor =" | |||
| width="200" height=" | |||
| width=" | |||
|- | |- | ||
| | | Aerobic Respiration | ||
| | | Aerobes and facultative anaerobes such as ''Staphylococcus'', ''[https://en.wikipedia.org/wiki/Nocardia Nocardia]'', ''[[Pseudomonas]]'' | ||
|- | |- | ||
| | | Denitrification | ||
| | | Facultative anaerobes such as ''[[Agrobacterium]]'', ''[[Alcaligenes]]'', ''[[Bacillus]]'', ''Paracoccus'', ''Micrococcus'' | ||
|- | |- | ||
| | | Manganese Reduction | ||
| | | ''[[Bacillus]]'', ''[[Geobacter]]'', ''[[Pseudomonas]]'', ''Shewanella'' | ||
|- | |- | ||
| | | Iron Reduction | ||
| | | ''[[Desulfovibrio]]'', ''[[Pseudomonas]]'', ''Geothrix'', ''Shewanella'', ''Thiobacillus'' | ||
|- | |- | ||
| | | Sulfate Reduction | ||
| | | Generally obligate anaerobes such as ''[[Desulfobacter]]'', ''[[Desulfococcus]]'', ''[[Desulfosarcina]]'', ''Desulfosporosinus'' | ||
|- | |- | ||
| | | Methanogenesis | ||
| | | [[Methanogens]] such as ''Methanobacterium'' and [https://en.wikipedia.org/wiki/Archaea Archaea] (different from bacteria) | ||
|} | |} | ||
[[Image:PseudomonasImage.jpg|300px|thumb|left|The ''Pseudomonas'' genus has a wide variety of metabolic capabilities among its species. [https://elmundodelavida.wordpress.com/category/ciencia/ Source]]] | |||
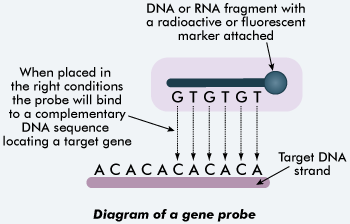
[[Image:Probes.gif|400px|thumb|center|[https://en.wikipedia.org/wiki/Hybridization_probe DNA and RNA probes] are used for identifying bacteria in samples. [http://tle.westone.wa.gov.au/content/file/969144ed-0d3b-fa04-2e88-8b23de2a630c/1/human_bio_science_3b.zip/content/005_dna/page_17.htm Source]]] | |||
== | ==Effects of Flooding on Soil Environment== | ||
===Mobility of Minerals and Gasses=== | |||
[[Image:Aggregation.jpg|200px|thumb|right|A) unaffected soil and B) soil incubated anaerobically. Flooding caused mobilization of organic matter and disaggregation, resulting in the larger grains (decreased stability). [https://dl.sciencesocieties.org/publications/sssaj/abstracts/73/2/550 Source]]] | |||
Water also acts as a solvent for ions and soluble compounds, thus increasing the mobility and availability of metal ions, nutrients, and minerals. As a result of these physical effects in flooded soils, microbial respiration will have improved access to water-soluble compounds such as nitrate, perchlorate, manganese/ferric iron, sulfate, and carbon dioxide to act as electron acceptors. | |||
The diffusion of oxygen is over 10000 times slower in water than it is in air at standard temperature and pressure, resulting in the replenishing rate being much slower in flooded soils. Carbon dioxide is also decreased but to a lesser degree due to it being far more soluble than oxygen. <ref>Greenway H, William A, Timothy DC. (2006). Conditions leading to high CO2 (>5 kPa) in waterlogged-flooded soils and possible effects on root growth and metabolism. Annals of Botany, 98, 9–32. Retrieved March 10, 2016 from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3291891/ .</ref> | |||
== | ===Flooded Soil Aggregate Structure=== | ||
Though some degree of moisture is important for aggregate formation and microbial activity, flooded soils exhibit decreased aggregate stability compared to unsaturated soils. Oxygen depletion and subsequent use of various elements for redox contributes to this decreased aggregation. Organic carbon is also made more soluble (like metals and minerals) under reducing conditions. The decreased stability of the soil is unlikely to recover due to drainage or volatilization of chemicals, removing them from the local environment. Since areas such as marshlands and rice fields are perpetually flooded, their soil’s aggregate stability rarely improves. <ref>De-Campos, A. B., Mamedov, A. I., & Huang, C. (2009). Short-Term Reducing Conditions Decrease Soil Aggregation. Soil Science Society of America Journal, 73(2), 550. Retrieved February 23, 2016, from https://dl.sciencesocieties.org/publications/sssaj/abstracts/73/2/550 .</ref> | |||
=== | ===Soil Gradients=== | ||
[[Image:Column.jpg|200px|thumb|left|A Winogradsky column. [http://beautyinscience.com/Biology.html Source] ]] | |||
The progression of electron acceptor utilization occurs at different rates in different layers of soil. A process like methanogenesis will occur earlier several feet underground than at the surface due to decreased access to other compounds. This will result in gradients at various depths of soil. These gradients will differ by pH, color, chemical prevalence, and microbial population. | |||
Since the soil is a more closed system (with less mobility of chemicals) than the surface, air, or bodies of water, gradients can be observed with a similar closed system: a Winogradsky column. This column, consisting of a sealed column of soil with provided organic material (such as an egg), allows for simulation of an anaerobic soil environment. <ref>Scientific American. (2013, September 19). Soil Science: Make a Winogradsky Column. Retrieved from http://www.scientificamerican.com/article/bring-science-home-soil-column/ </ref> | |||
---- | |||
=== | ===Variation of pH=== | ||
pH has a major influence over the dissolution and sorption of several important toxins and nutrients in the soil. Low pH values increase the solubility of free aluminum (Al<sup>3+</sup>) and iron (Fe<sup>3+</sup> and Fe<sup>2+</sup>) ions which can be toxic in high concentrations, while also reducing the availability of phosphorus. | |||
When soil is initially saturated with water, the pH drops due to the accumulation of carbonic acid formed from trapped carbon dioxide produced from respiration. Fermentation also contributes to pH decreasing through the production of organic acids. This is quickly followed by an increase of pH as hydrogen cations are consumed in microbially-driven redox reactions. The soil then will gradually approach and stabilize near a neutral pH, with pH increasing in acidic soil and decreasing in basic soil due to products such as carbonate forming a buffer. <ref>Kirk, G. J. (2004). The biogeochemistry of submerged soils. Chichester: Wiley. </ref> | |||
=== | ===Soil Gleying=== | ||
[[Image:gleyed.jpg|200px|thumb|right|Gray colors produced in gleyed soil. [http://wetland-delineation.rutgers.edu/87-wetland-delineation-manual/wetland-delineation-manual-part3.html Source] ]] | |||
Gleying is a phenomenon in which waterlogged soils are discolored by the color changes due to reduction of ferric iron into ferrous iron. Although ferric iron exists as an insoluble form in flooded soils, more ferrous iron can accumulate by the reduction of ferric iron over time. This results in a greenish, blue, grey soil color. In general Fe<sup>3+</sup> -reducing fermentative bacteria can be readily isolated from gleyed soils. The black color of soils/solution is frequently observed in flooded soil. This may result from the formation of iron sulfides (FeS) and pyrite (FeS2). <ref>Wenk, H., & Bulakh, A. G. (2004). Minerals: Their constitution and origin. Cambridge: Cambridge University Press. </ref> | |||
===Plant Nutrient Availability=== | |||
Flooded soils can have a direct impact on plants by preventing efficient gas exchange between the plant roots and the soil. These conditions also change the types of microbes that are active and what they produce. Flooded soils cause anaerobic conditions which forces many microbes to use less favorable electron acceptors. These less favorable acceptors could have also served as nutrition for the plants; this creates competition between the plants and the microbes in certain flooded soils. By using different electron acceptors the microbes will release different products causing a chemical change in the soil that can be toxic. <ref>Minamikawa, K., & Sakai, N. (2005). The effect of water management based on soil redox potential on methane emission from two kinds of paddy soils in Japan. Agriculture, Ecosystems & Environment, 107(4), 397-407. Retrieved from http://www.sciencedirect.com/science/article/pii/S0167880904002208 .</ref> | |||
===Flooded to Unflooded Conditions=== | |||
When waterlogged soils drain, oxygen diffuses into soil pores. The soil’s Eh increases and aerobic activity kicks in. At higher Eh zones ( > 500 mV), undecomposed soil organic matter is rapidly used as an electron donor by aerobes. With oxygen, many of the reduced products of anaerobic respiration can now be used as electron donors by lithotrophs. Manganese is oxidized back to MnO2 which gives some aerated soils a black color, and ferrous iron is oxidized by an iron-oxidizing bacteria, resulting in the formation of ferric oxides or ferric hydroxide minerals that give the soil a red, yellow, or brownish texture. <ref name="Richardson" /> | |||
==Environmental Issues== | |||
CH<sub>4</sub> | Flooded soils are dynamic ecosystems that play an important role in biogeochemical cycling and in the production of greenhouse gases. Methane (CH<sub>4</sub><sup>+</sup>) and nitrous oxide (N<sub>2</sub>O) are produced as byproducts of anaerobic metabolism in the low-redox zones characteristic of flooded soils, where oxygen is lacking. Carbon dioxide (CO<sub>2</sub>), which receives widespread attention as a greenhouse gas and potential source of global warming, may also be produced at the interface of anaerobic-aerobic zones through the consumption of methane gas. However, it should be noted that from a global standpoint methane and nitrous oxide on a per molecule basis have the potential to contribute 25x and 300x more to global warming over the next century than carbon dioxide, respectively. Thus the conversion of methane gas to carbon dioxide essentially reduces the greenhouse gas effect by 25x per molecule per 100 years. Although the number areas classified as wetlands has decreased in past years, the effect of flooded soils to the global climate is clear.<ref>Schlesinger, W. H., & Bernhardt, E. S. (2013). Biogeochemistry: An analysis of global change (3rd ed.). San Diego, CA: Academic Press. </ref> | ||
===Methane Production; Methanogenesis=== | ===Methane Production; Methanogenesis=== | ||
[[Image:Methane.jpg|thumb|300px|A natural source of methane gas]] | [[Image:Methane.jpg|thumb|300px|A natural source of methane gas]] | ||
Methane production occurs exclusively in anaerobic conditions by a group of Archaea known as methanogens. These microbes are obligatory, and require extremely low redox conditions in the range of -100mV | Methane production occurs exclusively in anaerobic conditions by a group of Archaea known as methanogens. These microbes are obligatory, and require extremely low redox conditions in the range of -100mV. If oxygen is introduced into the system, methanogenesis ceases; thus, the process of methanogenesis depends on saturated soil conditions.<ref name="Sylvia" /> | ||
Methanogenesis can occur via one of two pathways: either by 1) CO<sub>2</sub> reduction or by 2) acetate fermentation. | Methanogenesis can occur via one of two pathways: either by 1) CO<sub>2</sub> reduction or by 2) acetate fermentation. | ||
| Line 221: | Line 151: | ||
Both acetate and hydrogen are byproducts of anaerobic fermentation. | Both acetate and hydrogen are byproducts of anaerobic fermentation. | ||
Because the process of methanogenesis is “fed” byproducts produced from a complex series of degradation processes which are themselves “fed” complex organic matter, rates of methane production are highly sensitive to changes in temperature. Methanogenesis has a Q10 value in the range of 30-40, which is substantially higher than most biochemical process | Because the process of methanogenesis is “fed” byproducts produced from a complex series of degradation processes which are themselves “fed” complex organic matter, rates of methane production are highly sensitive to changes in temperature. Methanogenesis has a Q10 value in the range of 30-40, which is substantially higher than most biochemical process. | ||
Despite the clear effect of increasing temperatures on the rate of methanogenesis, the actual impact of global warming on methane production rates in wetlands and permafrost regions is highly unpredictable. Because methanogenesis requires anoxic conditions, any drying of flooded soil environments would both decrease methane production and increase methane oxidation, reducing overall methane emissions. Alternatively, warmer climates could increase growing seasons, which would increase methane emissions | Despite the clear effect of increasing temperatures on the rate of methanogenesis, the actual impact of global warming on methane production rates in wetlands and permafrost regions is highly unpredictable. Because methanogenesis requires anoxic conditions, any drying of flooded soil environments would both decrease methane production and increase methane oxidation, reducing overall methane emissions. Alternatively, warmer climates could increase growing seasons, which would increase methane emissions.<ref name="Sylvia" /> | ||
===CO<sub>2</sub> Production via Methane Consumption: Methanotrophy=== | ===CO<sub>2</sub> Production via Methane Consumption: Methanotrophy=== | ||
| Line 232: | Line 162: | ||
CH<sub>4</sub><sup>+</sup> + 2O<sub>2</sub> --> CO<sub>2</sub> + 2H<sub>2</sub>O | CH<sub>4</sub><sup>+</sup> + 2O<sub>2</sub> --> CO<sub>2</sub> + 2H<sub>2</sub>O | ||
Methane is similar in size and shape to ammonium; and there is some evidence that nitrifiers (ammonium oxidizers) can also oxidize methane | Methane is similar in size and shape to ammonium; and there is some evidence that nitrifiers (ammonium oxidizers) can also oxidize methane. Because they are molecularly similar, NH<sup>4</sup><sup>+</sup> competes at the enzyme’s active site, inhibiting methane oxidation. As a result, methanotrophy is generally inhibited by the addition of fertilizer or excess nitrogen in the system, when ammonium levels are high. | ||
Alternatively, if nitrogen is extremely limiting the addition of nitrogen will stimulate methanotrophy and actually increase methane consumption. So although it is generally expected that adding N-fertilizer will decrease CH<sub>4</sub><sup>+</sup> consumption and lead to increased global warming potential, sometime the opposite effect may occur. | Alternatively, if nitrogen is extremely limiting the addition of nitrogen will stimulate methanotrophy and actually increase methane consumption. So although it is generally expected that adding N-fertilizer will decrease CH<sub>4</sub><sup>+</sup> consumption and lead to increased global warming potential, sometime the opposite effect may occur.<ref name="Sylvia" /> | ||
===Nitrous Oxide; Denitrification=== | ===Nitrous Oxide; Denitrification=== | ||
| Line 249: | Line 179: | ||
Although a low redox potential is important for denitrification to occur (oxygen must not be present or it will “out-compete” nitrate as a terminal electron acceptor), redox requirements are not so low that this process cannot occur within anaerobic microsites of soil aggregates. | Although a low redox potential is important for denitrification to occur (oxygen must not be present or it will “out-compete” nitrate as a terminal electron acceptor), redox requirements are not so low that this process cannot occur within anaerobic microsites of soil aggregates. | ||
Factors affecting nitrous oxide production include oxygen, pH, and the ratio of nitrate to available C. Although denitrification rates decrease with increasing oxygen, the proportion of N evolved as nitrous oxide actually increases with increasing oxygen. Low pH generally inhibits the reduction of N<sub>2</sub>O to N<sub>2</sub>; thus at low pH, N<sub>2</sub>O will likely dominate. However, highly acidic soils have low N availability and low nitrification and denitrification rates. Thus, the highest rate of nitrous oxide production from denitrification occurs in moist soils that cycle N rapidly | Factors affecting nitrous oxide production include oxygen, pH, and the ratio of nitrate to available C. Although denitrification rates decrease with increasing oxygen, the proportion of N evolved as nitrous oxide actually increases with increasing oxygen. Low pH generally inhibits the reduction of N<sub>2</sub>O to N<sub>2</sub>; thus at low pH, N<sub>2</sub>O will likely dominate. However, highly acidic soils have low N availability and low nitrification and denitrification rates. Thus, the highest rate of nitrous oxide production from denitrification occurs in moist soils that cycle N rapidly.<ref name="Sylvia" /> | ||
==Current Research== | ==Current Research== | ||
Current research topics on the issue of flooded soils are heavily focused on greenhouse gas emissions produced as a result of the low redox conditions characteristic of these ecosystems. Other research topics may address impacts to plant growth, and chemical, physical, and biological aspects of flooded soils. | Current research topics on the issue of flooded soils are heavily focused on greenhouse gas emissions produced as a result of the low redox conditions characteristic of these ecosystems. Other research topics may address impacts to plant growth, and chemical, physical, and biological aspects of flooded soils. | ||
Many agricultural studies focus on rice paddies. Bacteria that oxidized acetate and reduced iron were identified and studied in flooded rice paddy soil through RNA probing.<ref>Hori, T., Müller, A., Igarashi, Y., Conrad, R., & Friedrich, M. W. (2009). Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. The ISME Journal ISME J, 4(2), 267-278. from http://www.nature.com/ismej/journal/v4/n2/full/ismej2009100a.html </ref> Another study observed factors affecting delivery of nitrogen to plants by microbial populations in flooded soils and found that the addition of sludge to soil facilitated nitrogen formation more than the addition of straw compost.<ref>El-Sharkawi, H. M. (2012). Effect of Nitrogen Sources on Microbial Biomass Nitrogen under Different Soil Types. ISRN Soil Science, 2012, 1-7. from http://www.citationmachine.net/bibliographies/77289062?new=true </ref> The effect of oxygen concentration on rates of reactions involving nitrogen, including nitrification and mineralization, in paddy soils was also studied.<ref>Yang, Y., Zhang, J., & Cai, Z. (2015). Nitrification activities and N mineralization in paddy soils are insensitive to oxygen concentration. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science, 66(3), 272-281. from http://www.tandfonline.com/doi/full/10.1080/09064710.2015.1093653 </ref> | |||
A review article was published in 2012, focusing on soil-plant interactions, including redox reactions’ effects on plant function, in wetlands.<ref>Pezeshki, S. R., & Delaune, R. D. (2012). Soil Oxidation-Reduction in Wetlands and Its Impact on Plant Functioning. Biology, 1(3), 196-221. from http://www.mdpi.com/2079-7737/1/2/196 </ref> | |||
[[Carbon cycle | Long-term retention of carbon in soil organic matter]] is an issue in frequently harvested soils. One study looked at various treatments of rice paddies and found that a combination of rice straw and inorganic fertilizer aided sequestration and led to “a higher grain yield”.<ref> Bhattacharyya, P., Roy, K., Neogi, S., Adhya, T., Rao, K., & Manna, M. (2012). Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil and Tillage Research, 124, 119-130. from http://www.sciencedirect.com/science/article/pii/S0167198712001195 </ref> | |||
A recent study discussed the fate of metals such as cadmium, nickel, and aluminum in impacted creeks in Kenya, Tanzania, and Mozambique.<ref>Kamau, J. N., Kuschk, P., Machiwa, J., Macia, A., Mothes, S., Mwangi, S., . . . Kappelmeyer, U. (2015). Investigating the distribution and fate of Al, Cd, Cr, Cu, Mn, Ni, Pb and Zn in sewage-impacted mangrove-fringed creeks of Kenya, Tanzania and Mozambique. J Soils Sediments Journal of Soils and Sediments, 15(12), 2453-2465. Retrieved from http://link.springer.com/article/10.1007/s11368-015-1214-3 </ref> | |||
Many other studies focus on the degradation of ground contaminants such as pesticides so that their results can be used in [[bioremediation]].<ref>Levén, L., Nyberg, K., & Schnürer, A. (2012). Conversion of phenols during anaerobic digestion of organic solid waste – A review of important microorganisms and impact of temperature. Journal of Environmental Management, 95. Retrieved from http://www.sciencedirect.com/science/article/pii/S0301479710003531 </ref> The fate of cycloxaprid, an agricultural insecticide, was observed in anaerobic conditions (including flooded soils). Radioisotopic tracing showed that various soil types had different effects on the chemical.<ref>Liu, X., Xu, X., Li, C., Zhang, H., Fu, Q., Shao, X., . . . Li, Z. (2016). Assessment of the environmental fate of cycloxaprid in flooded and anaerobic soils by radioisotopic tracing. Science of The Total Environment, 543, 116-122. Retrieved from http://www.sciencedirect.com/science/article/pii/S0048969715310007 </ref> | |||
Lastly, there is always the topic of greenhouse gas emissions. Arctic, temperate, and tropical regions were recently studied for measurements of greenhouse gas emissions and its relation to temperature.<ref>Turetsky, M. R., Kotowska, A., Bubier, J., Dise, N. B., Crill, P., Hornibrook, E. R., . . . Wilmking, M. (2014). A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Glob Change Biol Global Change Biology, 20(7), 2183-2197. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/gcb.12580/pdf </ref> Another group of researchers found a correlation between anaerobic methane oxidation in freshwater wetlands and reduced methane emissions.<ref>Segarra, K. E., Schubotz, F., Samarkin, V., Yoshinaga, M. Y., Hinrichs, K., & Joye, S. B. (2015). High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions. Nature Communications Nat Comms, 6, 7477. Retrieved from http://www.nature.com/ncomms/2015/150630/ncomms8477/full/ncomms8477.html </ref> | |||
(http://www. | |||
==References== | ==References== | ||
<references /> | <references /> | ||
Edited by students of [mailto:kmscow@ucdavis.edu Kate Scow] | Edited by students of [mailto:kmscow@ucdavis.edu Kate Scow] | ||
<!-- Do not edit or remove this line --> [[Category:Pages edited by students of Kate Scow at UC Davis]] | <!-- Do not edit or remove this line --> [[Category:Pages edited by students of Kate Scow at UC Davis]] | ||
Latest revision as of 07:26, 9 February 2018
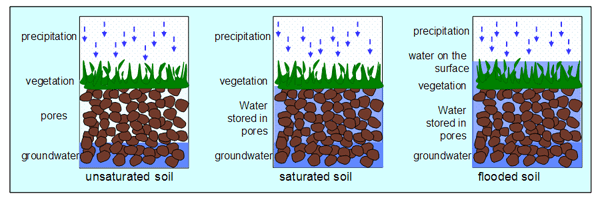

Flooded soils are a condition in which an area of soil is oversaturated with water, often due to natural occurrence or with intended purpose for agricultural reasons. Perpetually flooded soils can be found in wetlands, swamps and marshes; temporary flooded soils can be an effect of season weather or agricultural practices. As water levels fluctuate, and soils alternate between flooded and unflooded states, the chemical makeup of the soil environment will continuously change. A soil’s water content directly influences both inorganic and microbial reactions that affect the soil’s redox potential (Eh), acidity, alkalinity, and salinity.[1]
One of the most important effects of flooded soils is that the presence of oxygen is limited in such an environment, and any remaining oxygen is quickly used up via aerobic respiration. As a result, other compounds are used as electron acceptors in energy acquisition reactions; the microorganisms that specialize in conducting these other reactions are able to flourish and affect nutrient cycling in the ecosystem.
Microbial transformations of elements in anaerobic soils play a worldwide role in biogeochemical cycling of nutrients and in greenhouse gas emissions. Changes in the oxidation state of terminal electron acceptors may result in nutrient loss from the system via volatilization or leaching. Anaerobic microbial processes including denitrification, methanogenesis, and methanotrophy are responsible for releasing greenhouse gases (N2O, CH4, CO2) into the atmosphere. [2]
Key Microbial Processes
Microbial Respiration: Oxidation/Reduction Reactions
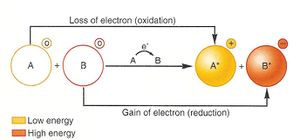
In order to obtain energy, many microbes make use of the process of respiration through an oxidation-reduction (redox) reaction. Respiration is a catabolic reaction that produces ATP in which either organic or inorganic compounds act as primary electron donors, and exogenous compounds act as the terminal electron acceptors. In a redox reaction, one molecule (the reducing agent) loses electrons and another molecule (the oxidizing agent) accepts electrons. Electron donors such as glucose, methanol, and NADH are energy sources that can be thought of as “giving up” their electrons, while another molecule is in need to receive said electrons. For example, in aerobic respiration, energy rich compounds like glucose (the reducing agent) are oxidized to carbon dioxide, with oxygen (the oxidizing agent) acting as a terminal electron acceptor and being reduced to water. In addition to oxygen, microorganisms use a large variety of electron acceptors.
Depending on the type of electron acceptors used by microorganisms, microbes can be placed into a variety of classifications. Strict aerobes can only use oxygen as a terminal electron acceptor. Obligate anaerobes cannot use oxygen and are actually inhibited or poisoned by oxygen. Facultative anaerobes are flexible in electron acceptor usage; as a result of this they can make use of other redox reactions to maintain a supply of energy as oxygen levels decrease.
Oxygen gas (O2) is one of the most favorable electron acceptors, but it is typically not available in flooded soils. Instead, facultative and strict anaerobic microbes utilize other oxidizing agents (electron acceptors) to carry out respiration. The amount of energy that can be obtained through respiration varies between compounds and microbes and will make use of these compounds in order of the decreasing redox potential, thus leading to a succession of acceptors.
Redox Potential (Eh)
Redox potential is the tendency for a reaction, specifically the movement and transfer of electrons, to occur spontaneously and is reported as Eh in mV. These measurements have been experimentally determined through aqueous solutions containing electrodes, one being the cathode (electron donator) and one being the anode (electron receiver). [4]
This voltage shows how likely the electrons will be moved in a solution. Redox potential is assigned individually to half-reactions (a single instance of oxidation or reduction), e.g. the Eh of the reduction of O2 to H2O will be different from that of the opposite oxidation of H2O to O2. Redox potential is also reported as standard reduction potential Eo. Reactions with a higher redox potential yield more net energy for the organism performing them, and this results in higher growth rates (in terms of population).
The Electron Tower
Microbes will successively use the highest energy yielding electron acceptors available in the order indicated on the electron tower, which is a ranking of common redox reactions by the amount of energy that can be obtained from them. Compounds are listed in redox pairs (oxidized form and reduced form) The greater the difference in electrical potential between the reactants and products of a reaction, the greater the release of energy that is crucial for microbial growth. [5]
O2, the lowest oxidizing agent on the tower, yields the most energy when reduced in a redox reaction with a specific electron donor and will be the first electron acceptor depleted when commonly available. In flooded soils, the amount of oxygen in the system will be very small. When the soil’s microbial population exhausts its remaining O2, it will begin using other available electron acceptors which provide the next highest amount of energy. Competition generally limits the use of weaker electron acceptors; for example, iron reducers (using Fe3+) may exist in the soil but will be dominated by the presence of denitrifiers, who will growth faster with access to their stronger electron acceptor (NO3-). Overall, this process of succession will continue as each electron acceptor supply is used.
Succession of Electron Acceptors
The main succession of electron acceptor usage in flooded soils is as follows:
Aerobic respiration: ½ O2 + 2e- + 2H+ -> H2O (by facultative anaerobes and aerobes)
Denitrification: 2NO3- + 12 H+ +10e- -> N2+6H2O (by denitrifiers)
Manganese reduction: MnO2 + 4H+ + 2e- ->Mn2+ + 2H2O (by manganese reducing bacteria)
Iron reduction: Fe(OH)3 + 3 H+ + 2e- -> Fe2+ + 2H2O (by iron reducing bacteria)
Sulfate reduction: SO42- + 10H+ +8e- -> H2S + 4H2O (by sulfate reducing bacteria)
Methane production: CO2 + 8 H+ + 8e- -> CH4 +2 H2O (by methanogens)
Nitrate Reduction
After O2, nitrate (NO3-) is one of the strongest electron acceptors as is represented in the electron tower. It can be obtained from transformations of other compounds containing nitrogen, such as ammonium (NH4+) and nitrite (NO2-). Denitrification reduces NO3- to nitrogen gas (N2) or various nitrogen oxides and is performed by facultative anaerobic microorganisms. Oxygen depletion is important for the nitrogen cycle as a whole, since if it were constantly present NO3- would be used at a much slower rate and contaminate soils through accumulation. [6]
Manganese Reduction
The next most energy-releasing electron acceptor after NO3- is manganese (IV) oxide (MnO2) which is is reduced to Mn2+ ions. In this form, manganese is very insoluble in water and forms masses in soils. Mn2+ is generally oxidized to this form in soils with a pH between 5 and 8, (as the rate increases with basicity). Many microorganisms that conduct this process are also capable of iron reduction, described below. [6]
Iron Reduction

The utilization of ferric iron ions (Fe3+), at approximately 120 mV, occurs when ions are released from metal deposits or minerals in the soil. Ferrous iron (Fe2+) product causes soil gleying, (a process described in a later section) when it accumulates. The source of the iron is also of relevance; whereas iron reduction in phosphate minerals can release phosphate for other organisms, it can also lead to corrosion of steel where iron reducers are present. Some Pseudomonas species can release pseudobactin, an iron-binding compound that limits its availability to other bacteria. [6]
Sulfate Reduction
Sulfate reduction begins occurring at 0 mV, and the dissimilatory reduction results in hydrogen sulfide (H2S) being released. However, H2S is prone to reaction with Fe2+ to form iron sulfide (FeS). As a result, it often reacts before it reaches the surface of the soil, unable to disperse into the air. [6] Sulfate reduction has several documented consequences, ranging from corrosion of underground iron pipes due to FeS formation and blackening of soil caused by liberation of organic matter. Hydrogen sulfide is known as “swamp gas”, due to its emergence from one form of soil flooding, and has an odor comparable to rotting eggs. It can accumulate in many bodies of water and the air above them; due to its flammability and toxicity, this is very dangerous. [7]
Methanogenesis (by Carbon Dioxide Reduction)
The process of using carbon dioxide (CO2) as a terminal electron acceptor results in the formation of methane (CH4) and is known as methanogenesis. In soil, methanogenesis occurs almost exclusively in a flooded condition due to its reduction potential being so low (below 100 mV). The use of CO2 in this fashion yields much less energy than the reactions of previous electron acceptors, so this process has lower growth rates in turn. Organisms who perform methanogenesis are known as methanogens and are a group of anaerobic Archaea. CH4 can also be produced as a result of acetate (CH3COOH) fermentation, which is also performed by methanogens. [6]
Fermentation in Flooded Soils (Non-Respiratory)
Fermentation is a different form of metabolism from respiration that occurs in the absence of a suitable terminal electron acceptor. Cells convert NADH and pyruvate from the glycolysis of sugars into NAD+ and other compounds, depending on the species that is fermenting. The various products of fermentation, including alcohols, lactic acid, and acetate are released into the surrounding soil and then become available for use by other anaerobic organisms. Additionally, fermentation generally reduces soil pH, which will encourage dissolution of minerals and their subsequent access by bacteria. [8]
Microorganisms Involved
As available oxygen declines, organisms that thrive under anoxic conditions proliferate using alternative electron acceptors. The order in which available electron acceptors are consumed can generally be predicted by the electron tower and associated energy yields of electron pairs. Changes in redox conditions of flooded soils over time reflects the successive availability of terminal electron acceptors from the electron tower, and will govern which microbes will thrive through being able to use them.
Some microbes below are able to oxidize the reduced form of their corresponding substance.[9]
| Process | Example Genera of Common Bacteria Involved |
| Aerobic Respiration | Aerobes and facultative anaerobes such as Staphylococcus, Nocardia, Pseudomonas |
| Denitrification | Facultative anaerobes such as Agrobacterium, Alcaligenes, Bacillus, Paracoccus, Micrococcus |
| Manganese Reduction | Bacillus, Geobacter, Pseudomonas, Shewanella |
| Iron Reduction | Desulfovibrio, Pseudomonas, Geothrix, Shewanella, Thiobacillus |
| Sulfate Reduction | Generally obligate anaerobes such as Desulfobacter, Desulfococcus, Desulfosarcina, Desulfosporosinus |
| Methanogenesis | Methanogens such as Methanobacterium and Archaea (different from bacteria) |
Effects of Flooding on Soil Environment
Mobility of Minerals and Gasses
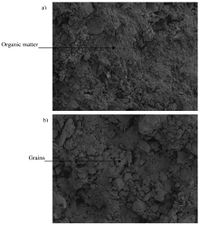
Water also acts as a solvent for ions and soluble compounds, thus increasing the mobility and availability of metal ions, nutrients, and minerals. As a result of these physical effects in flooded soils, microbial respiration will have improved access to water-soluble compounds such as nitrate, perchlorate, manganese/ferric iron, sulfate, and carbon dioxide to act as electron acceptors.
The diffusion of oxygen is over 10000 times slower in water than it is in air at standard temperature and pressure, resulting in the replenishing rate being much slower in flooded soils. Carbon dioxide is also decreased but to a lesser degree due to it being far more soluble than oxygen. [10]
Flooded Soil Aggregate Structure
Though some degree of moisture is important for aggregate formation and microbial activity, flooded soils exhibit decreased aggregate stability compared to unsaturated soils. Oxygen depletion and subsequent use of various elements for redox contributes to this decreased aggregation. Organic carbon is also made more soluble (like metals and minerals) under reducing conditions. The decreased stability of the soil is unlikely to recover due to drainage or volatilization of chemicals, removing them from the local environment. Since areas such as marshlands and rice fields are perpetually flooded, their soil’s aggregate stability rarely improves. [11]
Soil Gradients

The progression of electron acceptor utilization occurs at different rates in different layers of soil. A process like methanogenesis will occur earlier several feet underground than at the surface due to decreased access to other compounds. This will result in gradients at various depths of soil. These gradients will differ by pH, color, chemical prevalence, and microbial population.
Since the soil is a more closed system (with less mobility of chemicals) than the surface, air, or bodies of water, gradients can be observed with a similar closed system: a Winogradsky column. This column, consisting of a sealed column of soil with provided organic material (such as an egg), allows for simulation of an anaerobic soil environment. [12]
Variation of pH
pH has a major influence over the dissolution and sorption of several important toxins and nutrients in the soil. Low pH values increase the solubility of free aluminum (Al3+) and iron (Fe3+ and Fe2+) ions which can be toxic in high concentrations, while also reducing the availability of phosphorus.
When soil is initially saturated with water, the pH drops due to the accumulation of carbonic acid formed from trapped carbon dioxide produced from respiration. Fermentation also contributes to pH decreasing through the production of organic acids. This is quickly followed by an increase of pH as hydrogen cations are consumed in microbially-driven redox reactions. The soil then will gradually approach and stabilize near a neutral pH, with pH increasing in acidic soil and decreasing in basic soil due to products such as carbonate forming a buffer. [13]
Soil Gleying

Gleying is a phenomenon in which waterlogged soils are discolored by the color changes due to reduction of ferric iron into ferrous iron. Although ferric iron exists as an insoluble form in flooded soils, more ferrous iron can accumulate by the reduction of ferric iron over time. This results in a greenish, blue, grey soil color. In general Fe3+ -reducing fermentative bacteria can be readily isolated from gleyed soils. The black color of soils/solution is frequently observed in flooded soil. This may result from the formation of iron sulfides (FeS) and pyrite (FeS2). [14]
Plant Nutrient Availability
Flooded soils can have a direct impact on plants by preventing efficient gas exchange between the plant roots and the soil. These conditions also change the types of microbes that are active and what they produce. Flooded soils cause anaerobic conditions which forces many microbes to use less favorable electron acceptors. These less favorable acceptors could have also served as nutrition for the plants; this creates competition between the plants and the microbes in certain flooded soils. By using different electron acceptors the microbes will release different products causing a chemical change in the soil that can be toxic. [15]
Flooded to Unflooded Conditions
When waterlogged soils drain, oxygen diffuses into soil pores. The soil’s Eh increases and aerobic activity kicks in. At higher Eh zones ( > 500 mV), undecomposed soil organic matter is rapidly used as an electron donor by aerobes. With oxygen, many of the reduced products of anaerobic respiration can now be used as electron donors by lithotrophs. Manganese is oxidized back to MnO2 which gives some aerated soils a black color, and ferrous iron is oxidized by an iron-oxidizing bacteria, resulting in the formation of ferric oxides or ferric hydroxide minerals that give the soil a red, yellow, or brownish texture. [8]
Environmental Issues
Flooded soils are dynamic ecosystems that play an important role in biogeochemical cycling and in the production of greenhouse gases. Methane (CH4+) and nitrous oxide (N2O) are produced as byproducts of anaerobic metabolism in the low-redox zones characteristic of flooded soils, where oxygen is lacking. Carbon dioxide (CO2), which receives widespread attention as a greenhouse gas and potential source of global warming, may also be produced at the interface of anaerobic-aerobic zones through the consumption of methane gas. However, it should be noted that from a global standpoint methane and nitrous oxide on a per molecule basis have the potential to contribute 25x and 300x more to global warming over the next century than carbon dioxide, respectively. Thus the conversion of methane gas to carbon dioxide essentially reduces the greenhouse gas effect by 25x per molecule per 100 years. Although the number areas classified as wetlands has decreased in past years, the effect of flooded soils to the global climate is clear.[16]
Methane Production; Methanogenesis
Methane production occurs exclusively in anaerobic conditions by a group of Archaea known as methanogens. These microbes are obligatory, and require extremely low redox conditions in the range of -100mV. If oxygen is introduced into the system, methanogenesis ceases; thus, the process of methanogenesis depends on saturated soil conditions.[6]
Methanogenesis can occur via one of two pathways: either by 1) CO2 reduction or by 2) acetate fermentation.
1) CO2 + H2 --> CH4+ (CO2 reduction)
and
2) CH3COOH --> CH4+ + CO2 (acetate fermentation)
Both acetate and hydrogen are byproducts of anaerobic fermentation.
Because the process of methanogenesis is “fed” byproducts produced from a complex series of degradation processes which are themselves “fed” complex organic matter, rates of methane production are highly sensitive to changes in temperature. Methanogenesis has a Q10 value in the range of 30-40, which is substantially higher than most biochemical process.
Despite the clear effect of increasing temperatures on the rate of methanogenesis, the actual impact of global warming on methane production rates in wetlands and permafrost regions is highly unpredictable. Because methanogenesis requires anoxic conditions, any drying of flooded soil environments would both decrease methane production and increase methane oxidation, reducing overall methane emissions. Alternatively, warmer climates could increase growing seasons, which would increase methane emissions.[6]
CO2 Production via Methane Consumption: Methanotrophy
Some of the methane produced via methanogenesis in flooded soils may be consumed and oxidized to CO2 at the interface of the anaerobic-aerobic zones. This process occurs primarily by a group of bacteria known as methanotrophs. These microbes can be found in surface layers of wetland soils and unsaturated upland soils, and may be exposed to very high concentrations of methane gas, sometimes amounting to 10% or more of the dissolved gases. Methane is thought to be the only source of C and energy for these bacteria.
Methanotrophy occurs in the following reaction:
CH4+ + 2O2 --> CO2 + 2H2O
Methane is similar in size and shape to ammonium; and there is some evidence that nitrifiers (ammonium oxidizers) can also oxidize methane. Because they are molecularly similar, NH4+ competes at the enzyme’s active site, inhibiting methane oxidation. As a result, methanotrophy is generally inhibited by the addition of fertilizer or excess nitrogen in the system, when ammonium levels are high.
Alternatively, if nitrogen is extremely limiting the addition of nitrogen will stimulate methanotrophy and actually increase methane consumption. So although it is generally expected that adding N-fertilizer will decrease CH4+ consumption and lead to increased global warming potential, sometime the opposite effect may occur.[6]
Nitrous Oxide; Denitrification
Denitrification is an anaerobic process in which nitrate serves as the terminal electron acceptor, and generally some source of organic carbon is the electron donor (also H2 may serve as a donor).
In this process, nitrate is oxidized to nitric oxide, then nitrous oxide, and then fully oxidized to dinitrogen:
NO2- --> NO --> N2O --> N2
However, under certain conditions the full oxidation of NO3- to N2 does not occur and nitrous oxide (N2O) is produced.
Microbes responsible include both organotrophs and lithotrophs, and this process occurs primarily by facultative anaerobes.
Although a low redox potential is important for denitrification to occur (oxygen must not be present or it will “out-compete” nitrate as a terminal electron acceptor), redox requirements are not so low that this process cannot occur within anaerobic microsites of soil aggregates.
Factors affecting nitrous oxide production include oxygen, pH, and the ratio of nitrate to available C. Although denitrification rates decrease with increasing oxygen, the proportion of N evolved as nitrous oxide actually increases with increasing oxygen. Low pH generally inhibits the reduction of N2O to N2; thus at low pH, N2O will likely dominate. However, highly acidic soils have low N availability and low nitrification and denitrification rates. Thus, the highest rate of nitrous oxide production from denitrification occurs in moist soils that cycle N rapidly.[6]
Current Research
Current research topics on the issue of flooded soils are heavily focused on greenhouse gas emissions produced as a result of the low redox conditions characteristic of these ecosystems. Other research topics may address impacts to plant growth, and chemical, physical, and biological aspects of flooded soils.
Many agricultural studies focus on rice paddies. Bacteria that oxidized acetate and reduced iron were identified and studied in flooded rice paddy soil through RNA probing.[17] Another study observed factors affecting delivery of nitrogen to plants by microbial populations in flooded soils and found that the addition of sludge to soil facilitated nitrogen formation more than the addition of straw compost.[18] The effect of oxygen concentration on rates of reactions involving nitrogen, including nitrification and mineralization, in paddy soils was also studied.[19]
A review article was published in 2012, focusing on soil-plant interactions, including redox reactions’ effects on plant function, in wetlands.[20]
Long-term retention of carbon in soil organic matter is an issue in frequently harvested soils. One study looked at various treatments of rice paddies and found that a combination of rice straw and inorganic fertilizer aided sequestration and led to “a higher grain yield”.[21]
A recent study discussed the fate of metals such as cadmium, nickel, and aluminum in impacted creeks in Kenya, Tanzania, and Mozambique.[22]
Many other studies focus on the degradation of ground contaminants such as pesticides so that their results can be used in bioremediation.[23] The fate of cycloxaprid, an agricultural insecticide, was observed in anaerobic conditions (including flooded soils). Radioisotopic tracing showed that various soil types had different effects on the chemical.[24]
Lastly, there is always the topic of greenhouse gas emissions. Arctic, temperate, and tropical regions were recently studied for measurements of greenhouse gas emissions and its relation to temperature.[25] Another group of researchers found a correlation between anaerobic methane oxidation in freshwater wetlands and reduced methane emissions.[26]
References
- ↑ Dassonville, F., & Renault, P. (2002). Interactions between microbial processes and geochemical transformations under anaerobic conditions: A review. Agronomie, 22(1), 51-68. Retrieved from http://www.agronomy-journal.org/articles/agro/abs/2002/01/05/05.html.
- ↑ United States Environmental Protection Agency. (n.d.). Overview of Greenhouse Gases. Retrieved March 13, 2016, from http://www3.epa.gov/climatechange/ghgemissions/gases/ch4.html .
- ↑ Schüring, J., Schulz, H. D., & Fischer, W. R. (Eds.). (2000). Redox: Fundamentals, processes, and applications. New York City, NY: Springer.
- ↑ Redox Chemistry Primer. (n.d.). Retrieved March 12, 2016, from http://www.kgs.ku.edu/Hydro/GWtutor/Plume_Busters/remediate_refs/redox_chemistry.htm .
- ↑ Madigan, M. T., Martinko, J. M., & Parker, J. (2003). Brock biology of microorganisms (10th ed.). Upper Saddle River, NJ: Prentice Hall/Pearson Education.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 Silvia, D.M., et al. 2005. Principles and Applications of Soil Microbiology. 2nd ed. Pearson Prentice Hall, New Jersey.
- ↑ Occupational Safety & Health Administration. (n.d.). Safety and Health Topics | Hydrogen Sulfide. Retrieved February 23, 2016, from https://www.osha.gov/SLTC/hydrogensulfide/index.html .
- ↑ 8.0 8.1 Richardson, J. L., & Vepraskas, M. J. (2001). Wetland soils: Genesis, hydrology, landscapes, and classification. Boca Raton, FL: Lewis.
- ↑ Liesack, W. (2000). Microbiology of flooded rice paddies. FEMS Microbiology Reviews, 24(5), 625-645.
- ↑ Greenway H, William A, Timothy DC. (2006). Conditions leading to high CO2 (>5 kPa) in waterlogged-flooded soils and possible effects on root growth and metabolism. Annals of Botany, 98, 9–32. Retrieved March 10, 2016 from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3291891/ .
- ↑ De-Campos, A. B., Mamedov, A. I., & Huang, C. (2009). Short-Term Reducing Conditions Decrease Soil Aggregation. Soil Science Society of America Journal, 73(2), 550. Retrieved February 23, 2016, from https://dl.sciencesocieties.org/publications/sssaj/abstracts/73/2/550 .
- ↑ Scientific American. (2013, September 19). Soil Science: Make a Winogradsky Column. Retrieved from http://www.scientificamerican.com/article/bring-science-home-soil-column/
- ↑ Kirk, G. J. (2004). The biogeochemistry of submerged soils. Chichester: Wiley.
- ↑ Wenk, H., & Bulakh, A. G. (2004). Minerals: Their constitution and origin. Cambridge: Cambridge University Press.
- ↑ Minamikawa, K., & Sakai, N. (2005). The effect of water management based on soil redox potential on methane emission from two kinds of paddy soils in Japan. Agriculture, Ecosystems & Environment, 107(4), 397-407. Retrieved from http://www.sciencedirect.com/science/article/pii/S0167880904002208 .
- ↑ Schlesinger, W. H., & Bernhardt, E. S. (2013). Biogeochemistry: An analysis of global change (3rd ed.). San Diego, CA: Academic Press.
- ↑ Hori, T., Müller, A., Igarashi, Y., Conrad, R., & Friedrich, M. W. (2009). Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. The ISME Journal ISME J, 4(2), 267-278. from http://www.nature.com/ismej/journal/v4/n2/full/ismej2009100a.html
- ↑ El-Sharkawi, H. M. (2012). Effect of Nitrogen Sources on Microbial Biomass Nitrogen under Different Soil Types. ISRN Soil Science, 2012, 1-7. from http://www.citationmachine.net/bibliographies/77289062?new=true
- ↑ Yang, Y., Zhang, J., & Cai, Z. (2015). Nitrification activities and N mineralization in paddy soils are insensitive to oxygen concentration. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science, 66(3), 272-281. from http://www.tandfonline.com/doi/full/10.1080/09064710.2015.1093653
- ↑ Pezeshki, S. R., & Delaune, R. D. (2012). Soil Oxidation-Reduction in Wetlands and Its Impact on Plant Functioning. Biology, 1(3), 196-221. from http://www.mdpi.com/2079-7737/1/2/196
- ↑ Bhattacharyya, P., Roy, K., Neogi, S., Adhya, T., Rao, K., & Manna, M. (2012). Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil and Tillage Research, 124, 119-130. from http://www.sciencedirect.com/science/article/pii/S0167198712001195
- ↑ Kamau, J. N., Kuschk, P., Machiwa, J., Macia, A., Mothes, S., Mwangi, S., . . . Kappelmeyer, U. (2015). Investigating the distribution and fate of Al, Cd, Cr, Cu, Mn, Ni, Pb and Zn in sewage-impacted mangrove-fringed creeks of Kenya, Tanzania and Mozambique. J Soils Sediments Journal of Soils and Sediments, 15(12), 2453-2465. Retrieved from http://link.springer.com/article/10.1007/s11368-015-1214-3
- ↑ Levén, L., Nyberg, K., & Schnürer, A. (2012). Conversion of phenols during anaerobic digestion of organic solid waste – A review of important microorganisms and impact of temperature. Journal of Environmental Management, 95. Retrieved from http://www.sciencedirect.com/science/article/pii/S0301479710003531
- ↑ Liu, X., Xu, X., Li, C., Zhang, H., Fu, Q., Shao, X., . . . Li, Z. (2016). Assessment of the environmental fate of cycloxaprid in flooded and anaerobic soils by radioisotopic tracing. Science of The Total Environment, 543, 116-122. Retrieved from http://www.sciencedirect.com/science/article/pii/S0048969715310007
- ↑ Turetsky, M. R., Kotowska, A., Bubier, J., Dise, N. B., Crill, P., Hornibrook, E. R., . . . Wilmking, M. (2014). A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Glob Change Biol Global Change Biology, 20(7), 2183-2197. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/gcb.12580/pdf
- ↑ Segarra, K. E., Schubotz, F., Samarkin, V., Yoshinaga, M. Y., Hinrichs, K., & Joye, S. B. (2015). High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions. Nature Communications Nat Comms, 6, 7477. Retrieved from http://www.nature.com/ncomms/2015/150630/ncomms8477/full/ncomms8477.html
Edited by students of Kate Scow