Chlamydomonas reinhardtii
Classification
Domain: Eukaryota
Class: Chlorophyceae
Order: Chlamydomonadales
Family: Chlamydomonadaceae
Species
Chlamydomonas reinhardtii
Description and Significance
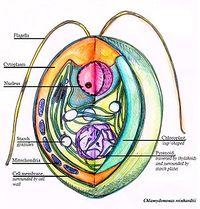
Chlamydomonas reinhardtii features are ovate in shape, about 10 um, unicellular with a distinct cell wall, and a single chloroplast in close proximity to the nucleus. The nucleus is typically located in the center and with a distinct nucleolus. There is an eyespot and one or several contractile vacuoles. One or several pyrenoids are located within the chloroplast and starch bodies surround the pyrenoid. Two anterior flagella are used for sensing and swimming. The mitochondria appears elongated, is bound by a double membrane and has distinct cristae protruding into the dark matrix [10].
Chlamydomonas are distributed throughout the world ranging from tropical, temperate regions. However, C. reinhardtii are specifically found in fresh-water lakes and ponds, in garden and farm soils [6,10]
Chlamydomonas provide an ideal model to study several mechanisms: 1) In photosynthesis, photosystem I and photosystem II, state transitions and its role in energy transfer [10]. 2) In motility, the coordination between the flagella and the photoreceptor in the eyespot allows an understanding of the signal transduction pathway [10, 25]. 3) In carbon assimilation during photosynthesis the pathways are like those of higher plants and so provide additional information [10]. 4) In the chloroplast, a fertile area for protein and vaccine production [17]. 5) Finally, C. reinhardtii and mutants employ novel methods to generate hydrogen gas and the synthesis of starch energy reserves needed in hydrogen gas production [23].
Genome Structure
C. reinhardtii are of high interest in genomics because of their ability to grow quickly with a 5 hour generation time and they are easy to grow on plates and in liquid media. The size of C. reinhardtii’s nuclear genome is 120 MB, and they are haploid with 17 linear chromosomes. During states of nitrogen deprivation, C. reinhardtii is capable of changing from haploid to diploid [1]
Considering C. reinhardtii is a very common model system, the amount of genetic data that is available is vast. There are currently three large integrated databases that contain information on the genetic loci, mutant alleles, sequenced genes, strain descriptions, nuclear genome sequences, chloroplast and mitochondrial gene sequences, gene model predictions, and more. These three databases include: ChlamyDB, ChlamyEST, and the JGI Chlamydomonas Genome Portal [9]
Cell Structure, Metabolism and Life Cycle
Chlamydomonas reinhardtii can grow autotrophically with CO2 as the carbon source, or heterotrophically by consuming acetate or mixotrophically when utilizing acetate as the carbon source while CO2 is assimilated during photosynthesis. As for the need for nitrogen, ammonium is the first choice for use and then other inorganic N compounds, as well as a few organic N compounds. Oxidized N forms of nitrate and nitrite, as inorganic forms are reduced to ammonium and easily assimilated. Phosphorous and sulfur are also needed. If sulfur is intentionally depleted in the cell, a metabolic switch changes the processes from aerobic photosynthetic growth to an anaerobic state while still in the light. After a day hydrogen gas begins to accumulate. [11, 21].
Hydrogen gas production has captivated the imaginations of numerous researchers because of the promise of a clean and non-polluting energy source. The best progress has been achieved with a manipulation of the Photosystem I, Photosystem II, screening for mutant hydrogenases that redirect electrons to drive higher (H2) production [3, 8]
Readers wanting more detailed information concerning hydrogen gas production are directed to: Improved Photobiological H2 production in engineered green algal cells [12]. Multiple facets of anoxic metabolism and hydrogen production in the unicellular green alga Chlamydomonas reinhardtii, [8]. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas, [20] Photobiotechnological hydrogen production with microalgae [16].
Sexual reproduction is initiated by low levels of nitrogen, either nitrate or ammonium and the presence of blue light. The phototropin, a photoreceptor protein located in the flagella, prevents chemotaxis --- no movement toward a nitrogen source that is out of the light. C. reinhardtii cells are heterothallic, mating only occurs with genetically dissimilar clones. Next, a mating structure appears that links filaments of both cells. Isogamy controls the pairing of gametes of identical size and structure. Glycoprotein molecules --- agglutinins--- ensure adhesion of the flagella of the opposite type, (+) and(-). Large groups clump together but soon individual pairs break away from the agglutinin substance. The flagella pairs come in close contact and protoplasts merge through a mating tube. The flagella move apart with one flagella of the pair (now called a zygote) controls the moving in the motile stage. Soon the zygotes are sessile and develop a spiny and thick wall made of glycoproteins and hydroxyproline. At this stage they are called hypnozygotes. After a short dormancy the zygotes undergo meiotic division yielding 4-8 haploid cells [6,7,29].
Asexual reproduction have parent cells generating 2, 4, 8, or 16 offspring cells via consecutive mitotic divisions. Divisions occur longitudinally with changes in the polarity of the following divisions [7].
Ecology
Chladmydomonas reinhardtii are photosynthetic and produce oxygen, however, they are also capable of producing H2 under anoxic conditions. At night, O2 concentrations can decrease because of respiration by other microbes. As a result, C. reinhardtii switches from an aerobic to fermentative metabolism, but the switch can be made under anoxic conditions in the light as well [4, 5, 14, 24]. The production of H2 has been of interest because of its potential use as a biofuel [18, 19]
Furthermore, at low CO2 levels, C. reinhardtii uses the extracellular enzyme carbonic anhydrase to catalyze the hydrolysis of carbonyl sulphide (COS) to CO2 and H2S. This is significant because carbonyl sulphide is the most abundant natural trace gas in the troposphere that can affect the earth’s radiation balance and climate [25].
C. reinhardtii also maintain a facultative mutualism with vitamin B12 (cobalamin)-producing bacteria. C. reinhardtii use cobalamin for growth, however, it has been found that vitamin B12-producing bacteria can increase C. reinhardtii fitness under heat stress as well. C. reinhardtii express vitamin B12-dependent (METH) and –independent (METE) methionine synthases, but when under heat stress, METE gene expression is inhibited along with repression of METE-mediated methione synthase activity subsequently [2, 12]. If vitamin B12-producing bacteria are present, METH gene expression can occur, allowing for methionine synthase activity to continue and keeping C. reinhardtii alive at increased temperatures [29] While it could be possible that the vitamin B12-producing bacteria could uptake carbon produced by C. reinhardtii, there has been no evidence of this occurrence [1]. Therefore, the relationship between C. reinhardtii and vitamin B12-producing bacteria is not fully understood.
References
[1] "Chlamydomonas Reinhardtii (ID 147) - Genome - NCBI". Ncbi.nlm.nih.gov. N.p., 2017. Web. 24 Apr. 2017.
[2] Droop MR. (2007). Vitamins, phytoplankton and bacteria: symbiosis or scavenging? J Plankton Res 29: 107–113.
[3] Esquível, Maria G., et al. "Efficient H 2 production via Chlamydomonas reinhardtii." Trends in biotechnology 29.12 (2011): 595-600. exhibit regulation. Environ Microbiol 14: 1466–1476.
[4] Gfeller RP, Gibbs M. 1984. Fermentative metabolism of Chlamydomonas reinhardtii: I. Analysis of fermentative products from starch in dark and light. Plant Physiology 75: 212–218
[5] Gibbs M, Gfeller RP, Chen C. 1986. Fermentative metabolism of Chlamydomonas reinhardii: III. Photoassimilation of acetate. Plant Physiology 82: 160–166.
[6] Goodenough, U. W., et al. "Molecular genetics of sexuality in Chlamydomonas." Annual review of plant biology 46.1 (1995): 21-44.
[7] Graham, Linda E, James M Graham, and Lee Warren Wilcox. Algae. 1st ed. San Francisco: Benjamin Cummings, 2009. Print.
[8] Grossman, Arthur R. et al. "Multiple Facets Of Anoxic Metabolism And Hydrogen Production In The Unicellular Green Alga Chlamydomonas Reinhardtii". New Phytologist 190.2 (2010): 279-288. Web.
[9] Grossman, Arthur R., et al. "Chlamydomonas reinhardtii at the crossroads of genomics." Eukaryotic cell 2.6 (2003): 1137-1150.
[10] Harris, Elizabeth H. The Chlamydomonas Sourcebook. 1st ed. Amsterdam [u.a.]: Elsevier, 2009. Print.
[11] Hemschemeier, Anja, et al. "Hydrogen production by Chlamydomonas reinhardtii: an elaborate interplay of electron sources and sinks." Planta 227.2 (2008): 397-407.
[12] Kazamia E, Czesnick H, Nguyen TTV, CroftMT, Sherwood E, Sasso S et al. (2012). Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria
[13] Kong, Qing-xue, et al. "Culture of microalgae Chlamydomonas reinhardtii in wastewater for biomass feedstock production." Applied biochemistry and Biotechnology 160.1 (2010): 9.
[14] Kreuzberg K. 1984. Starch fermentation via formate producing pathway in Chlamydomonas reinhardtii, Chlorogonium elongatum and Chlorella fusca.
[15] Kruse, Olaf, et al. "Improved photobiological H2 production in engineered green algal cells." Journal of Biological Chemistry 280.40 (2005): 34170-34177.
[16] Lehr, Florian, et al. "Process development for hydrogen production with Chlamydomonas reinhardtii based on growth and product formation kinetics." Journal of biotechnology 162.1 (2012): 89-96.
[17] Mayfield, Stephen P., Scott E. Franklin, and Richard A. Lerner. "Expression and assembly of a fully active antibody in algae." Proceedings of the National Academy of Sciences 100.2 (2003): 438-442.
[18] Melis A, Happe T. 2004. Trails of green alga hydrogen research – from Hans Gaffron to new frontiers. Photosynthesis Research 80: 401–409. A, Happe T. 2001. Hydrogen production. Green algae as a source of energy. Plant Physiology 127: 740–748.
[19] Melis, Anastasios, et al. "Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green AlgaChlamydomonas reinhardtii." Plant physiology 122.1 (2000): 127-136.
[20] Melis, Anastasios. "Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae)." Planta 226.5 (2007): 1075-1086.
[21] Merchant, Sabeeha S., et al. "The Chlamydomonas genome reveals the evolution of key animal and plant functions." Science 318.5848 (2007): 245-250.
[22] Mussgnug, Jan H., et al. "NAB1 is an RNA binding protein involved in the light-regulated differential expression of the light-harvesting antenna of Chlamydomonas reinhardtii." The Plant Cell 17.12 (2005): 3409-3421.
[23] 3S, Miyamoto K, Miura Y. 1987. Hydrogen evolution as a consumption mode of reducing equivalents in green algal fermentation.Plant Physiology 83: 1022–1026.
[24] Noriko, et al. "Phototactic activity in Chlamydomonas' non-phototactic'mutants deficient in Ca2+-dependent control of flagellar dominance or in inner-arm dynein." Journal of cell science 118.3 (2005): 529-537. Physiologia Plantarum 61: 87–94.
[25] Protoschill‐Krebs, G., C. Wilhelm, and J. Kesselmeier. "Consumption of carbonyl sulphide by Chlamydomonas reinhardtii with different activities of carbonic anhydrase (CA) induced by different CO2 growing regimes." Plant Biology 108.5 (1995): 445-448.
[26] Ramanan, Rishiram, et al. "Algae–bacteria interactions: evolution, ecology and emerging applications." Biotechnology advances 34.1 (2016): 14-29. Scientific, Oxford, 341 pp.
[27] Shrager, J. "Chlamydomonas Reinhardtii Genome Project. A Guide To The Generation And Use Of The Cdna Information". PLANT PHYSIOLOGY 131.2 (2003): 401-408. Web.
[28] South, G. R. & Whittick, A. 1987. Introduction to Phycology. Blackwell
[29] Xie, Bo, et al. "Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria." The ISME journal 7.8 (2013): 1544-1555.
Authors
Page authored by Catherine Kagemann and Harry Kaczorowski, students of Prof. Jay Lennon at Indiana University.
!["FIG. 5. Integration of databases. Data for an integrated C. reinhardtii database are gathered from ChlamyDB, ChlamyEST, the Chlamy database at JGI, and a variety of outside sources before being integrated in the relational database chado and served to users on the Internet. Links connecting ChlamyDB and JGI will be established to provide robust data retrieval [9]."](/images/thumb/9/94/Chlamydomonas_genome.jpg/600px-Chlamydomonas_genome.jpg)
