Chronic Salmonella Typhi Infection and Gallbladder Cancer: Difference between revisions
Hmoore2262 (talk | contribs) No edit summary |
Hmoore2262 (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
==<b>Introduction</b>== | ==<b>Introduction</b>== | ||
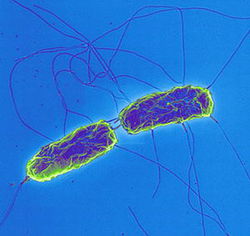
[[Image:salmonella_typhi.jpg|thumb| | [[Image:salmonella_typhi.jpg|thumb|250px|right|<b>Fig. 1.</b> <i>S.</i> Typhi bacteria, with rod shape and flagella, are shown. Copyright © Dr. Volker Brinkmann, Max Planck Institute for Infection Biology.]] | ||
<b>Chronic <i>S.</i> Typhi (<i>S. enterica enterica </i> serovar Typhi) infection is correlated with gallbladder cancer.</b> This rod shaped, flagellated, aerobic, Gram-negative bacterium is a pathogenic serovar of the <i>S. enterica enterica</i> subspecies. It is restricted to humans and reported to cause 21 million acute cases of typhoid fever annually, with 200,000 fatalities. | <b>Chronic <i>S.</i> Typhi (<i>S. enterica enterica </i> serovar Typhi) infection is correlated with gallbladder cancer.</b> This rod shaped, flagellated, aerobic, Gram-negative bacterium (Fig. 1) is a pathogenic serovar of the <i>S. enterica enterica</i> subspecies. It is restricted to humans and reported to cause 21 million acute cases of [http://www.cdc.gov/nczved/divisions/dfbmd/diseases/typhoid_fever/ typhoid fever] annually, with 200,000 fatalities. .<sup>1</sup> The bacteria invade the mucosal surface of the intestine but soon spread to deeper tissues such as liver, spleen, and bone marrow after macrophages phagocytose them.<sup>1</sup> Bacteria can also spread to the gallbladder via ducts from the liver.<sup>2</sup> | ||
A small percentage of the individuals who suffer an acute infection—about 3-5%—become asymptomatic carriers whose infections persist for many years following the acute illness. <i>S.</i> Typhi achieves this persistent carrier state by creating biofilms on | A small percentage of the individuals who suffer an acute infection—about 3-5%—become asymptomatic carriers whose infections persist for many years following the acute illness.<sup>7</sup> <i>S.</i> Typhi achieves this persistent carrier state by creating biofilms on cholesterol-based gallstones residing within the gallbladder. This carrier state requires both presence of cholesterol gallstones and <i>S.</i> Typhi infection of the gallbladder—so few of the individuals who suffer acute infection are at risk for chronic carriage.<sup>2</sup> Mary Mallon, or [http://en.wikipedia.org/wiki/Typhoid_Mary/ “Typhoid Mary”], was one well-known carrier who was both asymptomatic and highly contagious. The bacteria are transmitted between individuals—both acutely or chronically affected—primarily through fecal contamination of food or water.<sup>7</sup> | ||
This chronic typhoid carrier state can lead to chronic inflammation of the gallbladder, in which the bacteria metabolize primary bile acids to produce potentially carcinogenic toxins and metabolites. One such carcinogen is bacterial β-glucuronidase, | This chronic typhoid carrier state can lead to chronic inflammation of the gallbladder, in which the bacteria metabolize primary bile acids to produce potentially carcinogenic toxins and metabolites. One such carcinogen is bacterial β-glucuronidase, a glycosidase that, in addition to the other secondary bile acids that are produced from bacterial enzyme processing and concentration in the gallbladder, is mutagenic.<sup>4</sup> The result is carcinoma of the gallbladder epithelium.<sup>3</sup> One defining feature of the gallbladder is its efficacy in concentrating not only bile salts but also toxins—an effect that amplifies their mutagenic effects so that carcinoma develops here instead of in other organs in chronic <i>S.</i> Typhi infection. Epidemiologically, the chronic typhoid carrier state has been demonstrated to be the single most important risk factor for development of gallbladder cancer—surpassing an eightfold higher risk rate —in patients with cholesterol-based gallstones.<sup>3</sup> | ||
Epidemiologically, the chronic typhoid carrier state has been demonstrated to be the single most important risk factor for development of gallbladder | |||
<br> | <br> | ||
==<b>Role of cholesterol gallstones and biofilms in chronic <i>S.</i> Typhi carriage | ==<b>Key characteristics of the gallbladder and bile salts</b>== | ||
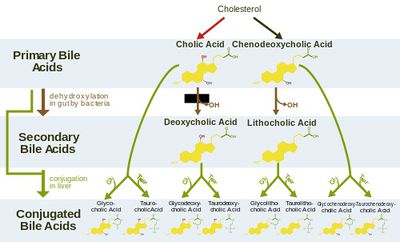
<i>S.</i> Typhi colonizes the gallbladder and persists in an asymptomatic carrier state.<sup>5</sup> This carriage is facilitated by formation of biofilms on cholesterol gallstones.<sup>6</sup> Crawford et al (2010) induced gallstones in mice then infected them and controls with <i>S.</i> Typhi. They found that significant amounts of <i>S.</i> Typhi could be recovered from the gallstones of mice with gallstones. These were induced by feeding with a lithogenic diet, which was supplemented with 1% cholesterol and 0.5% cholic acid. Bacteria could furthermore be recovered in stool samples of the mice with cholesterol gallstones—but not the control mice—for over a year. Significantly, it also took much longer for the mice with gallstones to recover from acute <i>S.</i> Typhi infection. Consistent results were obtained in vitro, affirming the specific role of cholesterol in this process.<sup>6</sup> | [[Image:bile_salt_chart.jpg|thumb|400px|right| <b>Fig. 2.</b> The flow chart demonstrates the structures and synthesis of secondary and conjugated bile acids from primary bile acids. Image reproduced under Creative Commons License.]] | ||
The gallbladder is an accessory organ that concentrates and stores the primary bile salts produced in the liver. Chemically speaking, primary bile salts mostly consist of variations of cholic and deoxycholic acids. They concentrated markedly in the gallbladder to levels exceeding 10-15% of total contents during storage.<sup>5</sup> | |||
Though bile is a detergent and generally toxic to bacteria in high concentrations, <i>S.</i> Typhi has unique resistance to this toxic detergent action of bile due in part to regulation by genes such as the PhoP-PhoQ virulence factor. In addition to activating and repressing the production of membrane and secreted proteins, this regulator has also been implicated in LPS modifications—all of which contribute to bile resistance in vivo. Two of the main bile acids implicated in this resistance are deoxycholic acid and conjugated forms of cholic and chenodeoxycholic acids. <sup>20</sup> | |||
<br> | |||
==<b>Fitness of <i>S.</i> Typhi in a gallbladder environment</b>== | |||
[[Image:LANGRIDGE2009--fig2.jpg|thumb|200px|left| <b>Fig. 3.</b> This genetic map showing the full <i>S.</i> Typhi genome was created with simultaneous assay. Genes are color coded for function; note in particular that genes related to bile tolerance are shown in brown.< <sup>21</sup>Image reproduced under Creative Commons License.]] | |||
===Role of cholesterol gallstones and biofilms in chronic <i>S.</i> Typhi carriage=== | |||
<i>S.</i> Typhi colonizes the gallbladder and persists in an asymptomatic carrier state.<sup>5</sup> This carriage is facilitated by formation of biofilms on cholesterol gallstones.<sup>6</sup> Crawford et al (2010) induced gallstones in mice then infected them and controls with <i>S.</i> Typhi. They found that significant amounts of <i>S.</i> Typhi could be recovered from the gallstones of mice with gallstones. This finding is demonstrated in Fig. 2, which clearly show the presence of bacteria on gallstones of infected organisms. These were induced by feeding with a lithogenic diet, which was supplemented with 1% cholesterol and 0.5% cholic acid. Bacteria could furthermore be recovered in stool samples of the mice with cholesterol gallstones—but not the control mice—for over a year.<sup>6</sup> This is due to the continuous shed of planktonic cells from the sessile, matrix-bound population and subsequent contamination of food and water, particularly in less developed countries.<sup>7</sup> Significantly, it also took much longer for the mice with gallstones to recover from acute <i>S.</i> Typhi infection. Consistent results were obtained in vitro, affirming the specific role of cholesterol in this process.<sup>6</sup> | |||
The <i>S.</i> Typhi O-antigen capsule is crucial to the specific binding affinity between the bacteria and cholesterol, and it is required for the biofilm formation in this environment. It is the key component of the exopolysaccharide (EPS) matrix, providing rigidity to the biofilm and also protecting the bacteria that it contains. <i>S.</i>The genetic material involved in the process of creating this capsule—<i>yihVW</i>, which is an operon that contains the <i>yihP</i> gene that codes for a symporter enzyme implicated in O antigen production—are upregulated when <i>S.</i> Typhi is grown in a concentrated bile environment. Thus, O-antigen capsule production is bile induced.<sup>7</sup> | |||
In the presence of bile, <i>S.</i> Typhi bacteria distribute into loose, multilayer matrices on cholesterol surfaces. These cells produce EPS components. On heterogeneous gallstones, <i>S.</i> Typhi does not cover the non-cholesterol surfaces, which further suggests binding specificity. Benefits conferred by biofilm formation include protection from antibiotic treatment and ability to remain in the gallbladder environment. Furthermore, strains that are unable to form the O-antigen have been demonstrated to be unsuccessful in biofilm formation.<sup>19</sup> Both O-antigen capsule and biofilm formation are thus crucial to successful adaptation to the gallbladder niche.<sup>8</sup> The O-antigen capsule has accordingly been identified as is a potential therapeutic target. <sup>7</sup> | |||
<br> | <br> | ||
==<b>Mechanisms of <i>S.</i> Typhi carcinogenicity in the gallbladder: how chronic carriage can lead to cancer</b>== | ==<b>Mechanisms of <i>S.</i> Typhi carcinogenicity in the gallbladder: how chronic carriage can lead to cancer</b>== | ||
===Bile | ===Bile mutates <i>S.</i> Typhi and increases fitness to the niche=== | ||
Bile salts are able to damage DNA, which has a mutagenic effect on <i>S.</i> Typhi bacteria that allows them to thrive in the gallbladder. <sup>9</sup> One proposed mechanism through <i>S.</i> Typhi is mutated relates to oxidative damage related to the conjugation that characterizes bile salt chemistry. | Bile salts are able to damage DNA, which has a mutagenic effect on <i>S.</i> Typhi bacteria that allows them to thrive in the gallbladder. <sup>9</sup> | ||
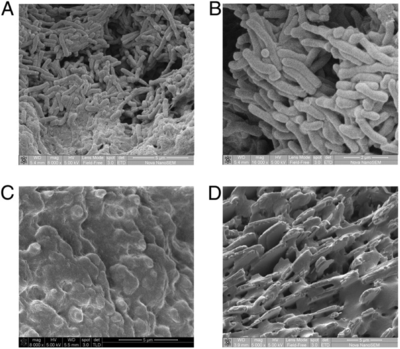
[[Image:gallstonestyphoid.png|thumb|400px|right| <b>Fig. 4.</b> Chronic <i>S.</i> Typhi biofilm formation is noted on cholesterol gallstones in the following collection of SEM images from Crawford et al (2010). (A) and (B) represent bacterial growth on cholesterol gallstones taken from infected controls. (C) shows a black, calcium bilirubinate gallstone without bacterial growth despite being taken from a <i>S.</i> Typhi carrier, demonstrating the specificity of cholesterol gallstones. (D) shows absence of biofilm in cholesterol gallstones from an uninfected patient.<sup>6</sup> Copyright © 2010 National Academy of Sciences, USA.]] | |||
One proposed mechanism through <i>S.</i> Typhi is mutated relates to oxidative damage related to the conjugation that characterizes bile salt chemistry. Deoxycholic and lithocholic acids, which have been linked to tumor genesis, are released during bile deconjugation of the primary bile acids A main gallbladder function is to concentrate bile. This provides <i>S.</i> Typhi with an increased concentration of possible substrate mutations. Thus, this factor could select for <i>S.</i> Typhi survival within the gallbladder niche environment through genome rearrangements and subsequent polymorphisms that select for changes resulting in increased fitness of <i>S.</i> Typhi in chronic carriers.<sup>9</sup> | |||
Analysis of the frequency of <i>S.</i> Typhi genome rearrangements post exposure to bile salts | Analysis of the frequency of <i>S.</i> Typhi genome rearrangements post exposure to bile salts | ||
shows that these mutations tend to occur in several common locations and have particular manifestations. Bile, particularly in very high concentrations, can act as a mutagen in vivo for <i>S.</i> Typhi for a variety of particular gene targets including DNA adenine methylase, which works to decrease bile sensitivity and reduce rate of mutation in normal bacteria. This can lead to further mutations. <sup>10</sup> | shows that these mutations tend to occur in several common locations and have particular manifestations. Bile, particularly in very high concentrations, can act as a mutagen in vivo for <i>S.</i> Typhi for a variety of particular gene targets including DNA adenine methylase, which works to decrease bile sensitivity and reduce rate of mutation in normal bacteria. This can lead to further mutations. <sup>10</sup> | ||
===<i>S.</i> Typhi metabolism of bile produces carcinogenic products=== | |||
Although bile is not generally characterized as a strong mutagen, the high concentration and long exposure time are demonstrated to contribute to mutagenic potential and lead to cancerous changes of the gallbladder epithelium.<sup>10</sup> Furthermore, by metabolizing these salts and also the cholesterol of the gallstones, <i>S.</i> Typhi bacteria themselves are capable of producing compounds that are mutagenic to the gallbladder epithelium.<sup>9</sup> | Although bile is not generally characterized as a strong mutagen, the high concentration and long exposure time are demonstrated to contribute to mutagenic potential and lead to cancerous changes of the gallbladder epithelium.<sup>10</sup> Furthermore, by metabolizing these salts and also the cholesterol of the gallstones, <i>S.</i> Typhi bacteria themselves are capable of producing compounds that are mutagenic to the gallbladder epithelium.<sup>9</sup> | ||
The increased deoxycholate levels have been reported to change the protein production of <i>S.</i> Typhi. <sup>20</sup> | |||
===Chemical mechanisms of <i>S.</i> Typhi mutagenic action=== | ===Chemical mechanisms of <i>S.</i> Typhi mutagenic action=== | ||
Bile salt metabolism produces carcinogenic compounds in long-term <i>S.</i> Typhi carriers. Chemical mechanisms have been proposed. For example, the action of the β-glucuronidase enzyme, which leads to deconjugation of conjugated toxins and bile acids in this niche | Bile salt metabolism produces carcinogenic compounds in long-term <i>S.</i> Typhi carriers. Chemical mechanisms have been proposed. For example, the action of the β-glucuronidase enzyme, which leads to deconjugation of conjugated toxins and bile acids in this niche, could render them carcinogenic to the host and present in high concentrations.<sup>4</sup> | ||
Another mechanism that leads to mutagenicity in the host cells depends on β-glucuronidase activity.<sup>12</sup> | <i>S.</i> Typhi produces three key genotoxic compounds with potentially carcinogenic roles: Cytolethal distending toxin B, CDT, and cytotoxic necrotizing factor 1. Cytolethal distending toxin B creates DNA lesions through DNAase activity. CDT, which also functions as a DNAase, also damages DNA, in addition to inhibiting the cell cycle. Cytotoxic necrotizing factor-1 blocks cytokines, thus leading to inflammation and inhibition of the cell cycle. It furthermore modifies proteins from the Rho family, which act in terminating transcription in prokaryotes<sup>4</sup> | ||
Another mechanism that leads to mutagenicity in the host cells depends on β-glucuronidase activity.<sup>12</sup> metabolizes bile salt substrates—a process that creates noncarcinogenic products but includes the production of a high-energy, active intermediate compound. This intermediate acts by binding to DNA and has mutagenic potential in human epithelial cells sup>13</sup> | |||
[[Image:gallbladdercancer.jpg|thumb|300px|left| <b>Fig. 5.</b> Gallbladder carcinoma is shown with H&E stain. Image reproduced under Creative Commons License.]] | |||
Another proposed mechanism of mutagenicity relates to interactions with the cholesterol that | Other bacterial enzymes found in <i>S.</i> Typhi could act on primary bile acids and produce carcinogenic secondary bile acids at very high concentrations. The main concern here is high concentration of biliary deoxycholate, which has correlated with gallbladder carcinoma patients.<sup>4</sup> | ||
form the structural basis of the gallstones. Bacteria that colonize the gut can not only alter bile salts to a secondary bile form but also convert the cholesterol itself into carcinogenic compounds, including cholesterol 5alpha,6alpha-epoxide.<sup>11</sup> Additional studies demonstrated that <i>S.</i> Typhi bacteria are capable of metabolizing | Another proposed mechanism of mutagenicity relates to interactions with the cholesterol that form the structural basis of the gallstones. Bacteria that colonize the gut can not only alter bile salts to a secondary bile form but also convert the cholesterol itself into carcinogenic compounds, including cholesterol 5alpha,6alpha-epoxide, which can cause cancerous changes in epithelial cells.<sup>11</sup> Additional studies demonstrated that <i>S.</i> Typhi bacteria are capable of metabolizing a primary bile acids to mutagenic cholic acid derivative forms in the presence of bile and cholesterol.<sup>12</sup> | ||
Other mechanisms including the <i>S.</i> Typhi genotoxin Cytolethal Distending Toxin B (CDT) may also play a role in this mutagenic and pathogenic process. This toxin is | Other mechanisms including the <i>S.</i> Typhi genotoxin Cytolethal Distending Toxin B (CDT) may also play a role in this mutagenic and pathogenic process. This toxin is Toxin is produced <i>S.</i> Typhi and dependent on CdtB, a DNAase homolog that is the functional unit necessary for CDT expression. Through this mechanism, <i>S.</i>Typhi is able to create DNA lesions in target cells—including, potentially, cells of human hosts.<sup>4,14</sup> | ||
<br> | |||
==<b>Epidemiological links between chronic <i>S.</i> Typhi carriage and gallbladder cancer</b>== | ==<b>Epidemiological links between chronic <i>S.</i> Typhi carriage and gallbladder cancer</b>== | ||
===From biochemistry to epidemiology=== | ===From biochemistry to epidemiology=== | ||
The relationship between chronic <i>S.</i> Typhi carriage and gallbladder cancer has been researched and characterized in studies conducted in sites worldwide, including sites such as India, Scotland, the United States, and Mexico. These data include the link among cholesterol-based gallstones, <i>S.</i> Typhi carriage, and biofilm presence. One major case-control study performed in India—an endemic typhoid region—found that gallbladder cancer patients had significantly higher incidences of <i>S.</i> Typhi than controls and cholelithiasis patients did, at 29.4%. Furthermore, the risk of developing gallbladder carcinomas in typhoid carriers was 8.47 times higher than it was in non-carriers.<sup>15</sup> Furthermore, as found in a study conducted in Mexico, typhoid carriage and biofilms were identified in 4.9% of surgically removed gallstones, but neither was present without the other—thus corroborating the idea that the mechanism of carriage is biofilm formation.<sup>6</sup> | The relationship between chronic <i>S.</i> Typhi carriage and gallbladder cancer has been researched and characterized in studies conducted in sites worldwide, including sites such as India, Scotland, the United States, and Mexico. These data include the link among cholesterol-based gallstones, <i>S.</i> Typhi carriage, and biofilm presence. One major case-control study performed in India—an endemic typhoid region—found that gallbladder cancer patients had significantly higher incidences of <i>S.</i> Typhi than controls and cholelithiasis patients did, at 29.4%. Furthermore, the risk of developing gallbladder carcinomas in typhoid carriers was 8.47 times higher than it was in non-carriers.<sup>15</sup> Furthermore, as found in a study conducted in Mexico, typhoid carriage and biofilms were identified in 4.9% of surgically removed gallstones, but neither was present without the other—thus corroborating the idea that the mechanism of carriage is biofilm formation.<sup>6</sup> | ||
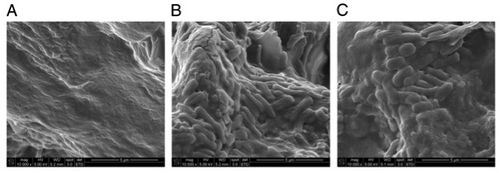
[[Image:Img1.jpg|thumb|500px|right| <b>Fig. 6.</b> Chronic <i>S.</i> Typhi biofilm formation is noted on cholesterol gallstones in the following collection of SEM images from Crawford et al (2010). (A) represents a gallstone taken from an uninfected control. (B) and (C) show bacterial biofilm growth on gallstones taken from organisms previously infected with <i>S.</i> Typhi.<sup>6</sup> Copyright © 2010 National Academy of Sciences, USA.]] | |||
The specificity of the niche gallbladder environment and the long time frame are key aspects of <i>S.</i> Typhi mutagenicity. A study conducted in Scotland thirty years following the 1964 typhoid outbreak found 16% of acutely infected individuals to be chronic carriers. Furthermore, these individuals were 167 times more likely to develop gallbladder cancer than were the patients who had suffered acute infections but not chronic carriage. Although risk of other cancers of digestive system organs was also elevated, this elevation was one to two orders of magnitude less intense by 1-2 orders on a logarithmic scale, thus stressing the specificity of gallbladder conditions.<sup>16</sup> An earlier study conducted with diverse American populations corroborates this specificity, suggesting that variations of bile salts acting as a carcinogen within the gallbladder, bile duct, and small bowel. The most marked finding, however, was that individuals identified as chronic typhoid carriers died of hepatobiliary cancer six times more often—a significant difference—than the control subjects did. <sup>17</sup> | The specificity of the niche gallbladder environment and the long time frame are key aspects of <i>S.</i> Typhi mutagenicity. A study conducted in Scotland thirty years following the 1964 typhoid outbreak found 16% of acutely infected individuals to be chronic carriers. Furthermore, these individuals were 167 times more likely to develop gallbladder cancer than were the patients who had suffered acute infections but not chronic carriage. Although risk of other cancers of digestive system organs was also elevated, this elevation was one to two orders of magnitude less intense by 1-2 orders on a logarithmic scale, thus stressing the specificity of gallbladder conditions.<sup>16</sup> An earlier study conducted with diverse American populations corroborates this specificity, suggesting that variations of bile salts acting as a carcinogen within the gallbladder, bile duct, and small bowel. The most marked finding, however, was that individuals identified as chronic typhoid carriers died of hepatobiliary cancer six times more often—a significant difference—than the control subjects did. <sup>17</sup> | ||
===Specificity of association cancer of the gallbladder as opposed to other pathologies=== | ===Specificity of association cancer of the gallbladder as opposed to other pathologies=== | ||
Bacterial degradation of primary bile acids in the gallbladder has been described be a factor in <i>S.</i> Typhi carcinogenesis in gallbladder carcinoma patients. One study found that, when patients with gallbladder carcinoma were compared to patients presenting only with gallstones, <i>S.</i> Typhi bacteria were identified in the bile of 40% of the gallbladder carcinoma patients and 30% of the cholelithiasis patients. However, cancer patients but not cholelithiasis patients had significantly elevated secondary bile acid levels—specifically, lithocholate and deoxycholate, which have both been linked to carcinogenicity in humans. Although gallbladder carcinoma patients consistently had higher secondary bile levels than cholelithiasis patients did regardless of <i>S.</i> Typhi carriage state, the cancer patients with bacteria in the bile had significantly higher secondary bile acid levels than the non-carrier cancer patients did.<sup>18</sup> | Bacterial degradation of primary bile acids in the gallbladder has been described be a factor in <i>S.</i> Typhi carcinogenesis in gallbladder carcinoma patients. One study found that, when patients with gallbladder carcinoma were compared to patients presenting only with gallstones, <i>S.</i> Typhi bacteria were identified in the bile of 40% of the gallbladder carcinoma patients and 30% of the cholelithiasis patients. However, cancer patients but not cholelithiasis patients had significantly elevated secondary bile acid levels—specifically, lithocholate and deoxycholate, which have both been linked extensively to carcinogenicity in humans. Although gallbladder carcinoma patients consistently had higher secondary bile levels than cholelithiasis patients did regardless of <i>S.</i> Typhi carriage state, the cancer patients with bacteria in the bile had significantly higher secondary bile acid levels than the non-carrier cancer patients did.<sup>18</sup> | ||
<br> | <br> | ||
==<b>Conclusion</b>== | ==<b>Conclusion</b>== | ||
| Line 40: | Line 49: | ||
<br> | <br> | ||
==<b>References</b>== | ==<b>References</b>== | ||
<sup>1</sup> Vladoianu, I.R., Chang, H.R. & Pechere, J. C. [http://www.ncbi.nlm.nih.gov/pubmed/2190062/ Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin.] Microb. Pathog. 8, 83–90 (1990). | <sup>1</sup> Vladoianu, I.R., Chang, H.R. & Pechere, J. C. [http://www.ncbi.nlm.nih.gov/pubmed/2190062/ Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin.] Microb. Pathog. 8, 83–90 (1990). | ||
| Line 69: | Line 77: | ||
<sup>14</sup> Haghjoo E, Galán JE. [http://www.ncbi.nlm.nih.gov/pubmed/15070766/ Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway.] Proc. Natl. Acad. Sci. USA 2004; 101: 4614-4619. | <sup>14</sup> Haghjoo E, Galán JE. [http://www.ncbi.nlm.nih.gov/pubmed/15070766/ Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway.] Proc. Natl. Acad. Sci. USA 2004; 101: 4614-4619. | ||
<sup>15</sup> Shukla VK, Singh H, Pandey M, et al (2000). [http://www.ncbi.nlm.nih.gov/pubmed/10795752/ Carcinoma of the gall | <sup>15</sup> Shukla VK, Singh H, Pandey M, et al (2000). [http://www.ncbi.nlm.nih.gov/pubmed/10795752/ Carcinoma of the gall bladder is it a sequel of typhoid?] Dig. Dis. Sci. 45:900-903. | ||
<sup>16</sup> Caygill C, Hill M, Braddick M, Sharp J (1994). [http://www.ncbi.nlm.nih.gov/pubmed/7903779/ Cancer mortality in chronic typhoid and paratyphoid carriers.] Lancet 343:83-84. | <sup>16</sup> Caygill C, Hill M, Braddick M, Sharp J (1994). [http://www.ncbi.nlm.nih.gov/pubmed/7903779/ Cancer mortality in chronic typhoid and paratyphoid carriers.] Lancet 343:83-84. | ||
| Line 76: | Line 84: | ||
<sup>18</sup> Pandey M, Vishwakarma RA, Khatri AK, et al (1995). [http://www.ncbi.nlm.nih.gov/pubmed/7723375/ Bile bacteria and gall bladder carcinogenesis.], J Surg. Oncol. 58:282-283. | <sup>18</sup> Pandey M, Vishwakarma RA, Khatri AK, et al (1995). [http://www.ncbi.nlm.nih.gov/pubmed/7723375/ Bile bacteria and gall bladder carcinogenesis.], J Surg. Oncol. 58:282-283. | ||
<sup>19</sup>Prouty AM, Schwesinger WH, Gunn JS (2002) [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC127943/ Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp.] Infect Immun 70: 2640–2649. | |||
<sup>20</sup>van Velkinburgh JC, Gunn JS. [http://iai.asm.org/content/67/4/1614.short/ PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp.] Infect. Immun. 1999; 67:1614–1622. [PubMed: 10084994] | |||
<sup>21</sup>Langridge GC, Phan MD, Turner DJ, Perkins TT, Parts L, et al. (2009) [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2792183/ Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants.] Genome Res 19: 2308–2316. | |||
Edited by Hannah Moore, a student of [http://www.jsd.claremont.edu/faculty/profile.asp?FacultyID=254/ Nora Sullivan] in BIOL187S (Microbial Life) in [http://www.jsd.claremont.edu/ The Keck Science Department of the Claremont Colleges] Spring 2013. | Edited by Hannah Moore, a student of [http://www.jsd.claremont.edu/faculty/profile.asp?FacultyID=254/ Nora Sullivan] in BIOL187S (Microbial Life) in [http://www.jsd.claremont.edu/ The Keck Science Department of the Claremont Colleges] Spring 2013. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Nora Sullivan at the Claremont Colleges]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Nora Sullivan at the Claremont Colleges]] | ||
Revision as of 06:53, 22 April 2013
Introduction
Chronic S. Typhi (S. enterica enterica serovar Typhi) infection is correlated with gallbladder cancer. This rod shaped, flagellated, aerobic, Gram-negative bacterium (Fig. 1) is a pathogenic serovar of the S. enterica enterica subspecies. It is restricted to humans and reported to cause 21 million acute cases of typhoid fever annually, with 200,000 fatalities. .1 The bacteria invade the mucosal surface of the intestine but soon spread to deeper tissues such as liver, spleen, and bone marrow after macrophages phagocytose them.1 Bacteria can also spread to the gallbladder via ducts from the liver.2
A small percentage of the individuals who suffer an acute infection—about 3-5%—become asymptomatic carriers whose infections persist for many years following the acute illness.7 S. Typhi achieves this persistent carrier state by creating biofilms on cholesterol-based gallstones residing within the gallbladder. This carrier state requires both presence of cholesterol gallstones and S. Typhi infection of the gallbladder—so few of the individuals who suffer acute infection are at risk for chronic carriage.2 Mary Mallon, or “Typhoid Mary”, was one well-known carrier who was both asymptomatic and highly contagious. The bacteria are transmitted between individuals—both acutely or chronically affected—primarily through fecal contamination of food or water.7
This chronic typhoid carrier state can lead to chronic inflammation of the gallbladder, in which the bacteria metabolize primary bile acids to produce potentially carcinogenic toxins and metabolites. One such carcinogen is bacterial β-glucuronidase, a glycosidase that, in addition to the other secondary bile acids that are produced from bacterial enzyme processing and concentration in the gallbladder, is mutagenic.4 The result is carcinoma of the gallbladder epithelium.3 One defining feature of the gallbladder is its efficacy in concentrating not only bile salts but also toxins—an effect that amplifies their mutagenic effects so that carcinoma develops here instead of in other organs in chronic S. Typhi infection. Epidemiologically, the chronic typhoid carrier state has been demonstrated to be the single most important risk factor for development of gallbladder cancer—surpassing an eightfold higher risk rate —in patients with cholesterol-based gallstones.3
Key characteristics of the gallbladder and bile salts
The gallbladder is an accessory organ that concentrates and stores the primary bile salts produced in the liver. Chemically speaking, primary bile salts mostly consist of variations of cholic and deoxycholic acids. They concentrated markedly in the gallbladder to levels exceeding 10-15% of total contents during storage.5
Though bile is a detergent and generally toxic to bacteria in high concentrations, S. Typhi has unique resistance to this toxic detergent action of bile due in part to regulation by genes such as the PhoP-PhoQ virulence factor. In addition to activating and repressing the production of membrane and secreted proteins, this regulator has also been implicated in LPS modifications—all of which contribute to bile resistance in vivo. Two of the main bile acids implicated in this resistance are deoxycholic acid and conjugated forms of cholic and chenodeoxycholic acids. 20
Fitness of S. Typhi in a gallbladder environment
Role of cholesterol gallstones and biofilms in chronic S. Typhi carriage
S. Typhi colonizes the gallbladder and persists in an asymptomatic carrier state.5 This carriage is facilitated by formation of biofilms on cholesterol gallstones.6 Crawford et al (2010) induced gallstones in mice then infected them and controls with S. Typhi. They found that significant amounts of S. Typhi could be recovered from the gallstones of mice with gallstones. This finding is demonstrated in Fig. 2, which clearly show the presence of bacteria on gallstones of infected organisms. These were induced by feeding with a lithogenic diet, which was supplemented with 1% cholesterol and 0.5% cholic acid. Bacteria could furthermore be recovered in stool samples of the mice with cholesterol gallstones—but not the control mice—for over a year.6 This is due to the continuous shed of planktonic cells from the sessile, matrix-bound population and subsequent contamination of food and water, particularly in less developed countries.7 Significantly, it also took much longer for the mice with gallstones to recover from acute S. Typhi infection. Consistent results were obtained in vitro, affirming the specific role of cholesterol in this process.6
The S. Typhi O-antigen capsule is crucial to the specific binding affinity between the bacteria and cholesterol, and it is required for the biofilm formation in this environment. It is the key component of the exopolysaccharide (EPS) matrix, providing rigidity to the biofilm and also protecting the bacteria that it contains. S.The genetic material involved in the process of creating this capsule—yihVW, which is an operon that contains the yihP gene that codes for a symporter enzyme implicated in O antigen production—are upregulated when S. Typhi is grown in a concentrated bile environment. Thus, O-antigen capsule production is bile induced.7
In the presence of bile, S. Typhi bacteria distribute into loose, multilayer matrices on cholesterol surfaces. These cells produce EPS components. On heterogeneous gallstones, S. Typhi does not cover the non-cholesterol surfaces, which further suggests binding specificity. Benefits conferred by biofilm formation include protection from antibiotic treatment and ability to remain in the gallbladder environment. Furthermore, strains that are unable to form the O-antigen have been demonstrated to be unsuccessful in biofilm formation.19 Both O-antigen capsule and biofilm formation are thus crucial to successful adaptation to the gallbladder niche.8 The O-antigen capsule has accordingly been identified as is a potential therapeutic target. 7
Mechanisms of S. Typhi carcinogenicity in the gallbladder: how chronic carriage can lead to cancer
Bile mutates S. Typhi and increases fitness to the niche
Bile salts are able to damage DNA, which has a mutagenic effect on S. Typhi bacteria that allows them to thrive in the gallbladder. 9
One proposed mechanism through S. Typhi is mutated relates to oxidative damage related to the conjugation that characterizes bile salt chemistry. Deoxycholic and lithocholic acids, which have been linked to tumor genesis, are released during bile deconjugation of the primary bile acids A main gallbladder function is to concentrate bile. This provides S. Typhi with an increased concentration of possible substrate mutations. Thus, this factor could select for S. Typhi survival within the gallbladder niche environment through genome rearrangements and subsequent polymorphisms that select for changes resulting in increased fitness of S. Typhi in chronic carriers.9 Analysis of the frequency of S. Typhi genome rearrangements post exposure to bile salts shows that these mutations tend to occur in several common locations and have particular manifestations. Bile, particularly in very high concentrations, can act as a mutagen in vivo for S. Typhi for a variety of particular gene targets including DNA adenine methylase, which works to decrease bile sensitivity and reduce rate of mutation in normal bacteria. This can lead to further mutations. 10
S. Typhi metabolism of bile produces carcinogenic products
Although bile is not generally characterized as a strong mutagen, the high concentration and long exposure time are demonstrated to contribute to mutagenic potential and lead to cancerous changes of the gallbladder epithelium.10 Furthermore, by metabolizing these salts and also the cholesterol of the gallstones, S. Typhi bacteria themselves are capable of producing compounds that are mutagenic to the gallbladder epithelium.9 The increased deoxycholate levels have been reported to change the protein production of S. Typhi. 20
Chemical mechanisms of S. Typhi mutagenic action
Bile salt metabolism produces carcinogenic compounds in long-term S. Typhi carriers. Chemical mechanisms have been proposed. For example, the action of the β-glucuronidase enzyme, which leads to deconjugation of conjugated toxins and bile acids in this niche, could render them carcinogenic to the host and present in high concentrations.4 S. Typhi produces three key genotoxic compounds with potentially carcinogenic roles: Cytolethal distending toxin B, CDT, and cytotoxic necrotizing factor 1. Cytolethal distending toxin B creates DNA lesions through DNAase activity. CDT, which also functions as a DNAase, also damages DNA, in addition to inhibiting the cell cycle. Cytotoxic necrotizing factor-1 blocks cytokines, thus leading to inflammation and inhibition of the cell cycle. It furthermore modifies proteins from the Rho family, which act in terminating transcription in prokaryotes4 Another mechanism that leads to mutagenicity in the host cells depends on β-glucuronidase activity.12 metabolizes bile salt substrates—a process that creates noncarcinogenic products but includes the production of a high-energy, active intermediate compound. This intermediate acts by binding to DNA and has mutagenic potential in human epithelial cells sup>13
Other bacterial enzymes found in S. Typhi could act on primary bile acids and produce carcinogenic secondary bile acids at very high concentrations. The main concern here is high concentration of biliary deoxycholate, which has correlated with gallbladder carcinoma patients.4
Another proposed mechanism of mutagenicity relates to interactions with the cholesterol that form the structural basis of the gallstones. Bacteria that colonize the gut can not only alter bile salts to a secondary bile form but also convert the cholesterol itself into carcinogenic compounds, including cholesterol 5alpha,6alpha-epoxide, which can cause cancerous changes in epithelial cells.11 Additional studies demonstrated that S. Typhi bacteria are capable of metabolizing a primary bile acids to mutagenic cholic acid derivative forms in the presence of bile and cholesterol.12
Other mechanisms including the S. Typhi genotoxin Cytolethal Distending Toxin B (CDT) may also play a role in this mutagenic and pathogenic process. This toxin is Toxin is produced S. Typhi and dependent on CdtB, a DNAase homolog that is the functional unit necessary for CDT expression. Through this mechanism, S.Typhi is able to create DNA lesions in target cells—including, potentially, cells of human hosts.4,14
Epidemiological links between chronic S. Typhi carriage and gallbladder cancer
From biochemistry to epidemiology
The relationship between chronic S. Typhi carriage and gallbladder cancer has been researched and characterized in studies conducted in sites worldwide, including sites such as India, Scotland, the United States, and Mexico. These data include the link among cholesterol-based gallstones, S. Typhi carriage, and biofilm presence. One major case-control study performed in India—an endemic typhoid region—found that gallbladder cancer patients had significantly higher incidences of S. Typhi than controls and cholelithiasis patients did, at 29.4%. Furthermore, the risk of developing gallbladder carcinomas in typhoid carriers was 8.47 times higher than it was in non-carriers.15 Furthermore, as found in a study conducted in Mexico, typhoid carriage and biofilms were identified in 4.9% of surgically removed gallstones, but neither was present without the other—thus corroborating the idea that the mechanism of carriage is biofilm formation.6
The specificity of the niche gallbladder environment and the long time frame are key aspects of S. Typhi mutagenicity. A study conducted in Scotland thirty years following the 1964 typhoid outbreak found 16% of acutely infected individuals to be chronic carriers. Furthermore, these individuals were 167 times more likely to develop gallbladder cancer than were the patients who had suffered acute infections but not chronic carriage. Although risk of other cancers of digestive system organs was also elevated, this elevation was one to two orders of magnitude less intense by 1-2 orders on a logarithmic scale, thus stressing the specificity of gallbladder conditions.16 An earlier study conducted with diverse American populations corroborates this specificity, suggesting that variations of bile salts acting as a carcinogen within the gallbladder, bile duct, and small bowel. The most marked finding, however, was that individuals identified as chronic typhoid carriers died of hepatobiliary cancer six times more often—a significant difference—than the control subjects did. 17
Specificity of association cancer of the gallbladder as opposed to other pathologies
Bacterial degradation of primary bile acids in the gallbladder has been described be a factor in S. Typhi carcinogenesis in gallbladder carcinoma patients. One study found that, when patients with gallbladder carcinoma were compared to patients presenting only with gallstones, S. Typhi bacteria were identified in the bile of 40% of the gallbladder carcinoma patients and 30% of the cholelithiasis patients. However, cancer patients but not cholelithiasis patients had significantly elevated secondary bile acid levels—specifically, lithocholate and deoxycholate, which have both been linked extensively to carcinogenicity in humans. Although gallbladder carcinoma patients consistently had higher secondary bile levels than cholelithiasis patients did regardless of S. Typhi carriage state, the cancer patients with bacteria in the bile had significantly higher secondary bile acid levels than the non-carrier cancer patients did.18
Conclusion
Long-term S. Typhi carriers tend to be asymptomatic—though highly contagious—and have a significantly elevated risk of developing gallbladder carcinoma. S. Typhi bacteria survive in the gallbladder niche by forming biofilms on cholesterol gallstones. The mutagenic effects of bile salts on S. Typhi further facilitate its survival, which is initially favored by structural components such as the O-antigen capsule, over long periods of time. Furthermore, S. Typhi itself has a mutagenic effect on the host through metabolism of bile salts into carcinogenic secondary bile compounds and other genotoxic effects. This connection has been characterized pathologically and epidemiologically by studies performed on a global scale, in which the chronic S. Typhi carrier state constitutes a key risk factor for gallbladder carcinoma.
References
1 Vladoianu, I.R., Chang, H.R. & Pechere, J. C. Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb. Pathog. 8, 83–90 (1990).
2 Hornick, R.B. et al. Typhoid fever: pathogenesis and immunologic control. N. Engl. J. Med. 283, 686–691 (1970).
3 Dutta U, Garg PK, Kumar R, Tandon RK (2000). Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am. J. Gastroenterol. 95:784-787.
4 Nath G, Gulati AK, Shukla VK (2010). Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J. Gastroenterol. 16:5395-5404.
5 Gonzalez-Escobedo G, Marshall JM, Gunn JS (2011). [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3255095/ Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nature Rev. Microbiol. 9:9-14.
6 Crawford RW, et al. (2010). Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc. Natl. Acad. Sci. U.S.A. 107:4353-4358.
7 Crawford RW, Gibson DL, Kay WW, Gunn JS (2008). Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect. Immun. 76:5341-5349.
8 Gibson DL, et al. 2006. Salmonella produces an O-antigen capsule regulated by AgfD and important for environmental persistence. J. Bacteriol. 188:7722–7730.
9 Prieto AI, Ramos-Morales F, Casadesús J (2006). Repair of DNA damage induced by bile salts in Salmonella enterica. Genetics. 174:575-584.
10 Prieto AI, Ramos-Morales F, Casadesús J (2004). Bile-induced DNA damage in Salmonella enterica. Genetics 168:1787-1794.
11 Chipman JK (1982). Bile as a source of potential reactive metabolites. Toxicology 25:99-111.
12 Connor TH, Forti GC, Sitra P, Legator MS (1979). Bile as a source of mutagenic metabolites produced in vivo and detected by Salmonella typhimurium. Environ. Mutagen., Vol. 1, ISS 3, 269-276.
13 Kinoshita N, Gelboin HV (1978). Beta-glucuronidase catalyzed hydrolysis of benzoapyrene-3-glucuronide and binding of DNA. Science 199:307-9.
14 Haghjoo E, Galán JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. USA 2004; 101: 4614-4619.
15 Shukla VK, Singh H, Pandey M, et al (2000). Carcinoma of the gall bladder is it a sequel of typhoid? Dig. Dis. Sci. 45:900-903.
16 Caygill C, Hill M, Braddick M, Sharp J (1994). Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 343:83-84.
17 Welton JC, Marr JS, Friedman SM (1979). Association between hepatobiliary cancer and typhoid carrier state. Lancet 313(8120):791-794.
18 Pandey M, Vishwakarma RA, Khatri AK, et al (1995). Bile bacteria and gall bladder carcinogenesis., J Surg. Oncol. 58:282-283.
19Prouty AM, Schwesinger WH, Gunn JS (2002) Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun 70: 2640–2649.
20van Velkinburgh JC, Gunn JS. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect. Immun. 1999; 67:1614–1622. [PubMed: 10084994] 21Langridge GC, Phan MD, Turner DJ, Perkins TT, Parts L, et al. (2009) Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res 19: 2308–2316.
Edited by Hannah Moore, a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.