Chronic Salmonella Typhi Infection and Gallbladder Cancer: Difference between revisions
Hmoore2262 (talk | contribs) No edit summary |
Hmoore2262 (talk | contribs) No edit summary |
||
| Line 25: | Line 25: | ||
Although bile is not generally characterized as a strong mutagen in an absolute sense, the high concentration and long exposure time associated with chronic carriage are demonstrated to contribute to mutagenic potential and lead to cancerous changes of the gallbladder epithelium.<sup>10</sup>(<b>Fig. 5</b>) By metabolizing primary bile salts and also the cholesterol of the gallstones, <i>S.</i> Typhi bacteria themselves are capable of producing compounds that are mutagenic to the gallbladder epithelium.<sup>9</sup> The increased deoxycholate levels have also been reported to change the protein production of <i>S.</i> Typhi. <sup>20</sup> | Although bile is not generally characterized as a strong mutagen in an absolute sense, the high concentration and long exposure time associated with chronic carriage are demonstrated to contribute to mutagenic potential and lead to cancerous changes of the gallbladder epithelium.<sup>10</sup>(<b>Fig. 5</b>) By metabolizing primary bile salts and also the cholesterol of the gallstones, <i>S.</i> Typhi bacteria themselves are capable of producing compounds that are mutagenic to the gallbladder epithelium.<sup>9</sup> The increased deoxycholate levels have also been reported to change the protein production of <i>S.</i> Typhi. <sup>20</sup> | ||
===Chemical mechanisms of <i>S.</i> Typhi mutagenic action=== | ===Chemical mechanisms of <i>S.</i> Typhi mutagenic action=== | ||
Chemical mechanisms have been proposed for the bile salt metabolism that produces carcinogenic compounds in long-term <i>S.</i> Typhi carriers. For example, the action of the β-glucuronidase enzyme, which can lead to deconjugation of conjugated toxins and bile acids, renders metabolites that are carcinogenic to the host and also present in high concentrations.<sup>4</sup>This enzyme metabolizes bile salt substrates—a process that creates noncarcinogenic products but includes the production of a high-energy, active intermediate compound. This intermediate acts by binding to DNA and has mutagenic potential in human epithelial cells sup>12,13</sup> | Chemical mechanisms have been proposed for the bile salt metabolism that produces carcinogenic compounds in long-term <i>S.</i> Typhi carriers. For example, the action of the β-glucuronidase enzyme, which can lead to deconjugation of conjugated toxins and bile acids, renders metabolites that are carcinogenic to the host and also present in high concentrations.<sup>4</sup>This enzyme metabolizes bile salt substrates—a process that creates noncarcinogenic products but includes the production of a high-energy, active intermediate compound. This intermediate acts by binding to DNA and has mutagenic potential in human epithelial cells <sup>12,13</sup> | ||
<i>S.</i> Typhi produces two other key genotoxic compounds with potentially carcinogenic roles: cytolethal distending toxin B (CdtB)—which is the functional unit of cytolethal distending toxin (CDT) and cytotoxic necrotizing factor 1. CdtB is a DNAase homolog that is the functional unit necessary for CDT expression. Through this mechanism, <i>S.</i>Typhi is able to create DNA lesions in target cells—including, potentially, cells of human hosts—that result in pathogenesis.<sup>4,14</sup> Cytotoxic necrotizing factor-1 blocks cytokines, thus leading to inflammation and inhibition of the cell cycle. It furthermore modifies proteins from the Rho family, which normally act to terminate transcription in prokaryotes<sup>4</sup> | <i>S.</i> Typhi produces two other key genotoxic compounds with potentially carcinogenic roles: cytolethal distending toxin B (CdtB)—which is the functional unit of cytolethal distending toxin (CDT) and cytotoxic necrotizing factor 1. CdtB is a DNAase homolog that is the functional unit necessary for CDT expression. Through this mechanism, <i>S.</i>Typhi is able to create DNA lesions in target cells—including, potentially, cells of human hosts—that result in pathogenesis.<sup>4,14</sup> Cytotoxic necrotizing factor-1 blocks cytokines, thus leading to inflammation and inhibition of the cell cycle. It furthermore modifies proteins from the Rho family, which normally act to terminate transcription in prokaryotes<sup>4</sup> | ||
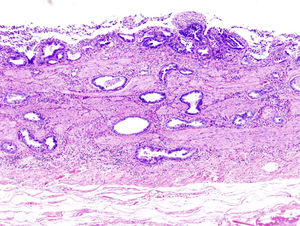
[[Image:gallbladdercancer.jpg|thumb|300px|left| <b>Fig. 5.</b> Gallbladder carcinoma is visualized with H&E stain. Image reproduced under Creative Commons License.]] | [[Image:gallbladdercancer.jpg|thumb|300px|left| <b>Fig. 5.</b> Gallbladder carcinoma is visualized with H&E stain. Image reproduced under Creative Commons License.]] | ||
Revision as of 09:15, 22 April 2013
Introduction
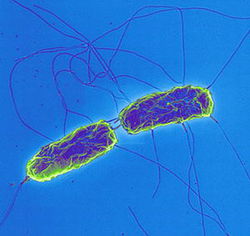
There is a strong correlation between chronic S. Typhi (S. enterica enterica serovar Typhi) infection and gallbladder cancer. S. Typhi, a rod shaped, flagellated, aerobic, Gram-negative bacterium (Fig. 1) is a pathogenic serovar of the S. enterica enterica subspecies. Its pathogenicity is restricted to humans and reported to cause 21 million acute cases of acute typhoid fever annually, with 200,000 fatalities.1 The bacteria invade the mucosal surface of the intestine but soon spread to deeper tissues such as liver, spleen, and bone marrow after phagocytosis by macrophages.1 They can also spread to the gallbladder via ducts from the liver during enterohepatic circulation.2
A small percentage of the individuals who suffer an acute infection—between 3 and 5%—become asymptomatic carriers whose infections persist for many years following the acute illness.7 S. Typhi achieves this persistent carrier state by creating biofilms on cholesterol-based gallstones residing within the gallbladders of infected individuals. This carrier state thus requires both presence of cholesterol gallstones and S. Typhi infection of the gallbladder—so few of the individuals who suffer acute infection are at risk for chronic carriage.2 The first described carrier was Mary Mallon, or “Typhoid Mary”, a food service worker who was both asymptomatic and highly contagious. These bacteria are transmitted among individuals—either acutely or chronically affected—primarily through fecal contamination of food or water.7
This carrier state can lead to chronic inflammation of the gallbladder, in which the bacteria metabolize primary bile acids to produce potentially carcinogenic toxins and metabolites. One such carcinogen producer is bacterial β-glucuronidase, a glycosidase that produces mutagenic compounds, which act in addition to the other secondary bile acids that are produced from bacterial enzyme processing and concentration in the gallbladder.4 The result is carcinoma of the gallbladder epithelium.3(Fig. 5). One defining feature of the gallbladder is its efficacy in concentrating not only bile salts but also toxins—an effect that amplifies their mutagenic effects. Thus, carcinoma develops here instead of in other organs that are implicated in chronic S. Typhi infection. Epidemiologically, the chronic typhoid carrier state has been demonstrated to be the single most important risk factor for development of gallbladder cancer—surpassing an eightfold increase—in patients with cholesterol-based gallstones.3
Key characteristics of the gallbladder and bile salts
The gallbladder is an accessory organ that concentrates and stores the primary bile salts produced in the liver. Chemically speaking, primary bile salts mostly consist of variations of cholic and deoxycholic acids. They are stored and concentrated markedly in the gallbladder to levels exceeding 10-15% of total gallbladder contents.5
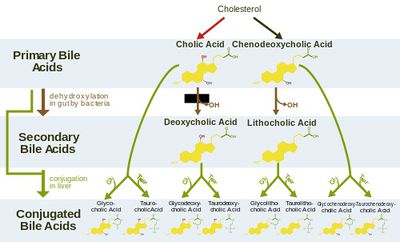
Though bile is a detergent and generally toxic to bacteria in such high concentrations, S. Typhi has unique resistance to this toxicity and detergent action. This is due in part to the protection provided by by genes such as the PhoP-PhoQ virulence factor. In addition to activating and repressing the production of both membrane and secreted proteins, this regulator has also been implicated in lipopolysaccharide (LPS) modifications—all of which contribute to a bile-resistant phenotype in vivo. This resistance applies to secondary bile acid products, which include deoxycholic acid and conjugated forms of cholic and chenodeoxycholic acids. 20(Fig. 2)
Fitness of S. Typhi in a gallbladder environment
===Role of cholesterol gallstones and biofilms in chronic S. Typhi carriage===
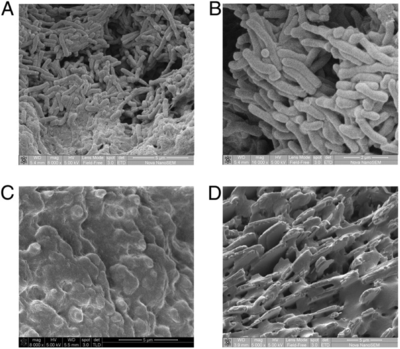
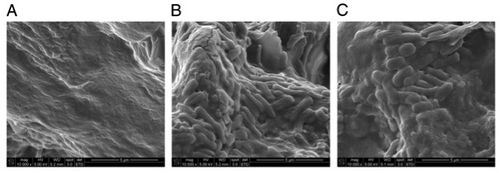
S. Typhi colonizes the gallbladder and persists in an asymptomatic carrier state.5 This carriage is facilitated by formation of biofilms on cholesterol gallstones.6 Significant amounts of S. Typhi can be recovered from cholesterol gallstones removed from infected individuals. SEM images clearly show the presence of bacteria on these gallstones (Fig. 4,6). These were induced experimentally by feeding mice with a lithogenic diet, which was supplemented with 1% cholesterol and 0.5% cholic acid. Furthermore, bacteria could furthermore be recovered from stool samples of infected mice with cholesterol gallstones—but not the control mice—for over a year.6 This is due to the continuous shedding of planktonic cells from the sessile, matrix-bound population. Epidemiologically speaking, this is the mechanism of contagion through subsequent contamination of food and water, particularly in less developed countries.7 Significantly, it also takes much longer for the mice with gallstones to recover from acute S. Typhi infection. Consistent results were obtained on a cholesterol substrate in vitro, affirming the specific role of cholesterol in this process.6
The O-antigen capsule is crucial to the specific binding affinity between S. Typhi bacteria and cholesterol, and it is required for biofilm formation. It is the key component of the exopolysaccharide (EPS) matrix, providing rigidity to the biofilm and also protecting the bacteria that it contains. S.The operon involved in the process of creating this capsule—yihVW, which contains the yihP gene that codes for a symporter enzyme implicated in O antigen production—is upregulated when S. Typhi is grown in a concentrated bile environment. Thus, O-antigen capsule production is not only crucial to biofilm production but also bile induced.7
In the presence of bile, S. Typhi bacteria form loose, multilayer matrices on cholesterol surfaces. These cells produce O-antigen and other EPS components. On heterogeneous gallstones, S. Typhi does not cover the non-cholesterol surfaces (Fig. 4), which further suggests binding specificity. Benefits conferred by biofilm formation include protection from antibiotic treatment and ability to tolerate the bile-rich gallbladder environment. Strains that are unable to form the O-antigen have been demonstrated to be unsuccessful in biofilm formation.19,8The O-antigen capsule has accordingly been identified as is a potential therapeutic target in other studies.7
Bile mutates S. Typhi to increase its fitness to the niche
Bile salts are able to damage DNA, which has a mutagenic effect on S. Typhi bacteria that contributes to their adaptation to the gallbladder niche. 9
One proposed mechanism through which S. Typhi is mutated relates to oxidative damage and the conjugation that characterizes bile salt chemistry. Deoxycholic and lithocholic acids, which have been linked to tumor genesis in human, are released during deconjugation of the primary bile acids (Fig. 2). S. Typhi, in a concentrated bile environment, has multiple protein alterations in response to bile and deoxycholic acid. 20(Fig. 3). S. Typhi survival within the gallbladder niche environment can also be selected through genome rearrangements and subsequent polymorphisms that select for changes resulting in increased fitness of S. Typhi in chronic carriers.9 Following exposure to bile salts, analysis of the frequency of S. Typhi genome rearrangements shows that these mutations tend to occur in several common locations and have characteristic manifestations. Particularly in very high concentrations, bile mutates S. Typhi in vivo for gene targets including DNA adenine methylase, which normally works to decrease bile sensitivity and reduce rate of mutation in S. Typhi bacteria. Its mutation can thus lead to further mutations. 10
S. Typhi metabolism of bile produces carcinogenic products
Although bile is not generally characterized as a strong mutagen in an absolute sense, the high concentration and long exposure time associated with chronic carriage are demonstrated to contribute to mutagenic potential and lead to cancerous changes of the gallbladder epithelium.10(Fig. 5) By metabolizing primary bile salts and also the cholesterol of the gallstones, S. Typhi bacteria themselves are capable of producing compounds that are mutagenic to the gallbladder epithelium.9 The increased deoxycholate levels have also been reported to change the protein production of S. Typhi. 20
Chemical mechanisms of S. Typhi mutagenic action
Chemical mechanisms have been proposed for the bile salt metabolism that produces carcinogenic compounds in long-term S. Typhi carriers. For example, the action of the β-glucuronidase enzyme, which can lead to deconjugation of conjugated toxins and bile acids, renders metabolites that are carcinogenic to the host and also present in high concentrations.4This enzyme metabolizes bile salt substrates—a process that creates noncarcinogenic products but includes the production of a high-energy, active intermediate compound. This intermediate acts by binding to DNA and has mutagenic potential in human epithelial cells 12,13 S. Typhi produces two other key genotoxic compounds with potentially carcinogenic roles: cytolethal distending toxin B (CdtB)—which is the functional unit of cytolethal distending toxin (CDT) and cytotoxic necrotizing factor 1. CdtB is a DNAase homolog that is the functional unit necessary for CDT expression. Through this mechanism, S.Typhi is able to create DNA lesions in target cells—including, potentially, cells of human hosts—that result in pathogenesis.4,14 Cytotoxic necrotizing factor-1 blocks cytokines, thus leading to inflammation and inhibition of the cell cycle. It furthermore modifies proteins from the Rho family, which normally act to terminate transcription in prokaryotes4
Other bacterial enzymes found in S. Typhi act on primary bile acids to produce carcinogenic secondary bile acids with high concentrations. Another main concern here is high concentration of biliary deoxycholate, a secondary bile acid with elevated levels in gallbladder carcinoma patients.4
Another proposed mechanism of S. Typhi mutagenicity relates to interactions with the cholesterol that form the structural basis of the gallstones. These bacteria can not only alter bile salts to a secondary bile form but also convert the cholesterol itself into carcinogenic compounds, including cholesterol 5alpha,6alpha-epoxide, which causes cancerous changes in epithelial cells.11 Additional studies demonstrate that S. Typhi bacteria are capable of metabolizing primary bile acids to mutagenic cholic acid derivative forms in the presence of bile and cholesterol substrates.12
Epidemiological links between chronic S. Typhi carriage and gallbladder cancer
The relationship between chronic S. Typhi carriage and gallbladder cancer has been researched and characterized in sites worldwide, including both endemic and non-endemic typhoid regions such as India, Scotland, the United States, and Mexico. These data establish a link among cholesterol-based gallstones, S. Typhi carriage, and biofilm presence. One major case-control study performed in India—an endemic typhoid region—found that gallbladder cancer patients had significantly higher incidences of S. Typhi than controls and cholelithiasis patients did, at 29.4%. Furthermore, the risk of developing gallbladder carcinomas in these typhoid carriers was 8.47 times higher than it was in non-carriers.15 Furthermore, as found in a study conducted in Mexico, typhoid carriage and biofilms were identified in 4.9% of surgically removed gallstones, but neither was present without the other—thus affirming that the mechanism of carriage is biofilm formation.6
The specificity of the niche gallbladder environment and the long infection time are key aspects of S. Typhi mutagenicity. A study conducted in Scotland thirty years following the 1964 typhoid outbreak found 16% of acutely infected individuals to be chronic carriers. Furthermore, these individuals were 167 times more likely to develop gallbladder cancer than were the patients who had suffered acute infections but not chronic carriage. Although risk of other cancers of digestive system organs was also elevated in this population, this elevation was one to two orders of magnitude less intense by 1-2 orders on a logarithmic scale, thus stressing the specificity of carcinogenic conditions to the gallbladder.16 An earlier study conducted with diverse American populations corroborates this specificity, suggesting furthermore that variations of bile salts act as carcinogens within the gallbladder, bile duct, and small bowel. The most marked finding, however, was that individuals identified as chronic typhoid carriers died of hepatobiliary cancer six times more often—a significant difference—than the control subjects did. 17
Specificity of association cancer of the gallbladder as opposed to other pathologies
S. Typhi degradation of primary bile acids in the gallbladder is a factor in carcinogenesis in gallbladder carcinoma patients. When patients with gallbladder carcinoma are compared to patients presenting only with gallstones, S. Typhi bacteria are identified in the bile of 40% of gallbladder carcinoma patients and 30% of cholelithiasis patients in one study conducted in India. However, cancer patients but not cholelithiasis patients tend to have significantly elevated secondary bile acid levels—specifically, lithocholate and deoxycholate, which are both linked extensively to carcinogenicity in humans.18
Conclusion
Long-term S. Typhi carriers are uncommon and asymptomatic but have a significantly elevated risk of developing gallbladder carcinoma. S. Typhi bacteria survive in the gallbladder niche by forming biofilms on cholesterol gallstones. The mutagenic effects of bile salts on S. Typhi further facilitate its survival, which is initially favored by structural components such as the O-antigen capsule, over long periods of time. Finally, S. Typhi itself has a mutagenic effect on the host through metabolism of bile salts into carcinogenic secondary bile compounds and other genotoxins. This connection has been characterized pathologically and epidemiologically by studies performed worldwide, in which the chronic S. Typhi carrier state constitutes a key risk factor for gallbladder carcinoma.
References
1 Vladoianu, I.R., Chang, H.R. & Pechere, J. C. Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb. Pathog. 8, 83–90 (1990).
2 Hornick, R.B. et al. Typhoid fever: pathogenesis and immunologic control. N. Engl. J. Med. 283, 686–691 (1970).
3 Dutta U, Garg PK, Kumar R, Tandon RK (2000). Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am. J. Gastroenterol. 95:784-787.
4 Nath G, Gulati AK, Shukla VK (2010). Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J. Gastroenterol. 16:5395-5404.
5 Gonzalez-Escobedo G, Marshall JM, Gunn JS (2011). [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3255095/ Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nature Rev. Microbiol. 9:9-14.
6 Crawford RW, et al. (2010). Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc. Natl. Acad. Sci. U.S.A. 107:4353-4358.
7 Crawford RW, Gibson DL, Kay WW, Gunn JS (2008). Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect. Immun. 76:5341-5349.
8 Gibson DL, et al. 2006. Salmonella produces an O-antigen capsule regulated by AgfD and important for environmental persistence. J. Bacteriol. 188:7722–7730.
9 Prieto AI, Ramos-Morales F, Casadesús J (2006). Repair of DNA damage induced by bile salts in Salmonella enterica. Genetics. 174:575-584.
10 Prieto AI, Ramos-Morales F, Casadesús J (2004). Bile-induced DNA damage in Salmonella enterica. Genetics 168:1787-1794.
11 Chipman JK (1982). Bile as a source of potential reactive metabolites. Toxicology 25:99-111.
12 Connor TH, Forti GC, Sitra P, Legator MS (1979). Bile as a source of mutagenic metabolites produced in vivo and detected by Salmonella typhimurium. Environ. Mutagen., Vol. 1, ISS 3, 269-276.
13 Kinoshita N, Gelboin HV (1978). Beta-glucuronidase catalyzed hydrolysis of benzoapyrene-3-glucuronide and binding of DNA. Science 199:307-9.
14 Haghjoo E, Galán JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. USA 2004; 101: 4614-4619.
15 Shukla VK, Singh H, Pandey M, et al (2000). Carcinoma of the gall bladder is it a sequel of typhoid? Dig. Dis. Sci. 45:900-903.
16 Caygill C, Hill M, Braddick M, Sharp J (1994). Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 343:83-84.
17 Welton JC, Marr JS, Friedman SM (1979). Association between hepatobiliary cancer and typhoid carrier state. Lancet 313(8120):791-794.
18 Pandey M, Vishwakarma RA, Khatri AK, et al (1995). Bile bacteria and gall bladder carcinogenesis., J Surg. Oncol. 58:282-283.
19Prouty AM, Schwesinger WH, Gunn JS (2002) Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun 70: 2640–2649.
20van Velkinburgh JC, Gunn JS. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect. Immun. 1999; 67:1614–1622. [PubMed: 10084994] 21Langridge GC, Phan MD, Turner DJ, Perkins TT, Parts L, et al. (2009) Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res 19: 2308–2316.
Edited by Hannah Moore, a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.