Citricoccus nitrophenolicus: Difference between revisions
No edit summary |
|||
| (15 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
[[Category:Pages edited by students of Dr.Ned Walker at Michigan State University]] | |||
==Classification== | ==Classification== | ||
| Line 21: | Line 23: | ||
|} | |} | ||
''Citricoccus nitrophenolicus'' | ''Citricoccus nitrophenolicus'' | ||
==Genome Structure== | ==Genome Structure== | ||
When studies were first being conducted on the Citricoccus genome, it was hard to come up with any particular information. But eventually, there was a draft genome sequence, CH26A, isolated from the Cuatro Ciénegas Basin (CCB) in Coahuila, Mexico. The CH26A genome was extracted from an environment was consistent with the actinomycete metabolism, which made it a lot easier to compare and somewhat understand the adaptive and evolutionary aspects of Citricoccus. | |||
Using the 16S rRNA gene phylogenetic analysis, a similarity between the CH26A and Citricoccus was drawn and the estimated size was 3.7 Mb, with a mean GC content of 70.96%. | |||
When further studies were conducted it was shown that that the presence of genes for both, the glycolytic and pentose phosphate pathways in addition to amino acid and nucleotide biosynthesis. | |||
==Metabolism and Life Cycle== | ==Metabolism and Life Cycle== | ||
C. nitrophenolicus is a strict aerobe and grows best with pNP as its sole carbon, electron and energy source. Although the favored food source was pNP, growth was also observed when food sources such as short chain fatty acids, salicylate and a range of alcohols such as phenols and 4-cholorphenols were present. All these served as electron donors for growth. | |||
- When pNP was used as the only source of carbon and energy: | |||
Metabolism of pNP resulted in the release of nitrite | |||
- When incubated with pNP and acetate: | |||
pNP was metabolized first and then acetate | |||
- When grown solely on acetate: | |||
Both nitrite and nitrate served as oxygen sources where nitrate was quickly reduced to nitrite and therefore quickly accumulated in cultures during aerobic growth. | |||
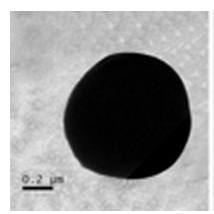
[[File:Tem.png|thumb|alt=A transmission electron micrograph.|C.nitrophenolicus]] | |||
==Ecology and Pathogenesis== | |||
pNP is a a highly stable compound found mainly in pesticides. Being highly stable, pNP is therefore highly soluble in water, thus easily penetrating the soil and polluting the environment. pNP is also listed as a "priority pollutant" on the US Environmental Protection Agency's List. | |||
Case Study: Breakwater 42, Denmark | |||
Problem: | |||
Parathions, a main pollutants of the Breakwater 42 waste dump, situated on a beach in North Western Denmark, leaked and polluted the beach and a near by bird sanctuary. | |||
Efforts taken: | |||
2 previous remediation efforts have excavated and the following was found: | |||
~6,000 tonnes of pollution and contaminated sand | |||
~270 tonnes of pollution is still located underground, in an estimated volume of 90,000m3, out of which parathions constitute ~225 tonnes of the pollution bound to the soil. | |||
Treating the remaining pollution: | |||
- Combination of alkaline hydrolysis and bioremediation | |||
--> Implementing both at the same time is bound to have a faster effect and more efficient removal | |||
-Alkaline hydrolysis will raise the pH to 11-12 | |||
--> Thereby converting many complex toxic substances to smaller, less toxic substances | |||
---> For example: pNP, a product of alkaline hydrolysis of parathions, is x1000 times more soluble than parathions | |||
- Bioremediation by either in situ or ex situ | |||
-->In situ: biostimulation or bioaugmentation | |||
-->Ex situ: pumping hydrolyzed chemicals into a bioreactor | |||
C. nitrophenolicus Role: | |||
- Due to the high pH post alkaline hydrolysis, it is desirable to find bacteria that can degrade chemicals at such high pH, thus reducing costs of neutralization | |||
- Due to it ability to degrade pNP and degrade it at pH values between 6.8 – 10 C. nitrophenolicus is the ideal microbe. | |||
Problem: | |||
- The pH levels post alkaline hydrolysis is between 11 and 12 | |||
Citricoccus nitrophenolicus can do the job, it just takes longer | |||
At pH of 10 it takes 3 times longer to degrade pNP than at pH 8! | |||
And resulted in a lower growth yield | |||
Solution: use of small volumes of acid to adjust to desirable pH | |||
==References== | ==References== | ||
[http://link.springer.com/article/10.1007/s10482-010-9513-6#page-1 Nielsen, Marie Bank, Kasper Urup Kjeldsen, and Kjeld Ingvorsen. "Description of Citricoccus nitrophenolicus sp. nov., a para-nitrophenol degrading actinobacterium isolated from a wastewater treatment plant and emended description of the genus Citricoccus Altenburger et al. 2002." Antonie van Leeuwenhoek 99.3 (2011): 489-499..] | |||
[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3194908/ Hayano-Kanashiro, Corina, et al. "First draft genome sequence of a strain from the genus Citricoccus." Journal of bacteriology 193.21 (2011): 6092-6093.] | |||
| Line 51: | Line 95: | ||
http://ijsb.sgmjournals.org/content/49/3/1157.full.pdf+html [This is where I found all the above info, I will properly cite within the next day or two] | http://ijsb.sgmjournals.org/content/49/3/1157.full.pdf+html [This is where I found all the above info, I will properly cite within the next day or two] | ||
==Author== | ==Author== | ||
Page authored by Cheryl Christie and Neha Rao, | Page authored by Cheryl Christie and Neha Rao, students of Dr.Ned Walker at Michigan State University. | ||
Latest revision as of 14:42, 14 August 2013
Classification
Kingdom - Bacteria
Phylum - Actinobacteria
Class - Actinobacteridae
Order - Actinomycetales
Family - Micrococcineae
Genus - Micrococcaceae
Species
|
NCBI: Taxonomy |
Citricoccus nitrophenolicus
Genome Structure
When studies were first being conducted on the Citricoccus genome, it was hard to come up with any particular information. But eventually, there was a draft genome sequence, CH26A, isolated from the Cuatro Ciénegas Basin (CCB) in Coahuila, Mexico. The CH26A genome was extracted from an environment was consistent with the actinomycete metabolism, which made it a lot easier to compare and somewhat understand the adaptive and evolutionary aspects of Citricoccus. Using the 16S rRNA gene phylogenetic analysis, a similarity between the CH26A and Citricoccus was drawn and the estimated size was 3.7 Mb, with a mean GC content of 70.96%. When further studies were conducted it was shown that that the presence of genes for both, the glycolytic and pentose phosphate pathways in addition to amino acid and nucleotide biosynthesis.
Metabolism and Life Cycle
C. nitrophenolicus is a strict aerobe and grows best with pNP as its sole carbon, electron and energy source. Although the favored food source was pNP, growth was also observed when food sources such as short chain fatty acids, salicylate and a range of alcohols such as phenols and 4-cholorphenols were present. All these served as electron donors for growth.
- When pNP was used as the only source of carbon and energy: Metabolism of pNP resulted in the release of nitrite
- When incubated with pNP and acetate: pNP was metabolized first and then acetate
- When grown solely on acetate: Both nitrite and nitrate served as oxygen sources where nitrate was quickly reduced to nitrite and therefore quickly accumulated in cultures during aerobic growth.
Ecology and Pathogenesis
pNP is a a highly stable compound found mainly in pesticides. Being highly stable, pNP is therefore highly soluble in water, thus easily penetrating the soil and polluting the environment. pNP is also listed as a "priority pollutant" on the US Environmental Protection Agency's List.
Case Study: Breakwater 42, Denmark
Problem: Parathions, a main pollutants of the Breakwater 42 waste dump, situated on a beach in North Western Denmark, leaked and polluted the beach and a near by bird sanctuary.
Efforts taken: 2 previous remediation efforts have excavated and the following was found: ~6,000 tonnes of pollution and contaminated sand ~270 tonnes of pollution is still located underground, in an estimated volume of 90,000m3, out of which parathions constitute ~225 tonnes of the pollution bound to the soil.
Treating the remaining pollution:
- Combination of alkaline hydrolysis and bioremediation
--> Implementing both at the same time is bound to have a faster effect and more efficient removal
-Alkaline hydrolysis will raise the pH to 11-12 --> Thereby converting many complex toxic substances to smaller, less toxic substances ---> For example: pNP, a product of alkaline hydrolysis of parathions, is x1000 times more soluble than parathions
- Bioremediation by either in situ or ex situ -->In situ: biostimulation or bioaugmentation -->Ex situ: pumping hydrolyzed chemicals into a bioreactor
C. nitrophenolicus Role:
- Due to the high pH post alkaline hydrolysis, it is desirable to find bacteria that can degrade chemicals at such high pH, thus reducing costs of neutralization
- Due to it ability to degrade pNP and degrade it at pH values between 6.8 – 10 C. nitrophenolicus is the ideal microbe.
Problem: - The pH levels post alkaline hydrolysis is between 11 and 12 Citricoccus nitrophenolicus can do the job, it just takes longer At pH of 10 it takes 3 times longer to degrade pNP than at pH 8! And resulted in a lower growth yield Solution: use of small volumes of acid to adjust to desirable pH
References
http://ijsb.sgmjournals.org/content/49/3/1157.full.pdf+html [This is where I found all the above info, I will properly cite within the next day or two]
Author
Page authored by Cheryl Christie and Neha Rao, students of Dr.Ned Walker at Michigan State University.